Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Pembrolizumab — extra information
Treatments Lesions (cancerous)
Pembrolizumab
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet Chief Editor in Chief: Adjunct A/Prof Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand. First published December 2014; updated March 2018. Copy edited by Gus Mitchell/Maria McGivern.
This article was updated with financial support from Merck Sharp & Dohme (New Zealand) Limited, distributors of Keytruda® in New Zealand. Sponsorship does not influence content.
Introduction
Benefits in melanoma patients
How it works
How to use
Potential drug interactions
Adverse effects
Use in particular populations
What is pembrolizumab?
Pembrolizumab (formerly known as lambrolizumab; trade name Keytruda®) is a drug marketed by Merck and Co (New Jersey, USA) that targets the programmed cell death 1 (PD-1) receptor. The drug is intended for use in treating metastatic melanoma.
On 4 September, 2014, the US Food and Drug Administration (FDA) approved pembrolizumab as a breakthrough therapy for the treatment of metastatic melanoma. It was approved for use for advanced melanoma in New Zealand in September 2015 and was funded by the NZ Pharmaceutical Management Agency (PHARMAC) for some patients with unresectable or metastatic melanoma from September 2016. Pembrolizumab is also used for metastatic non-small cell lung carcinoma, some cases of Hodgkin lymphoma, urothelial carcinoma (March 2018) and cutaneous squamous cell carcinoma (FDA, June 2020). Pembrolizumab is under investigation in other malignancies. It has been reported to have some effect in metastatic Merkel cell carcinoma.
Metastatic melanoma
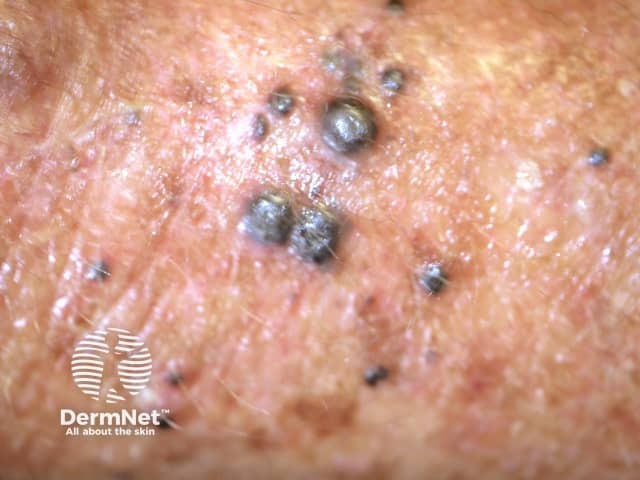
Cutaneous melanoma
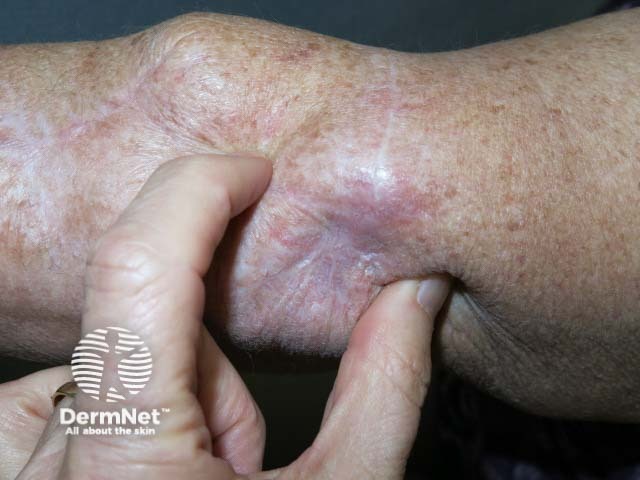
Cutaneous melanoma mestastases
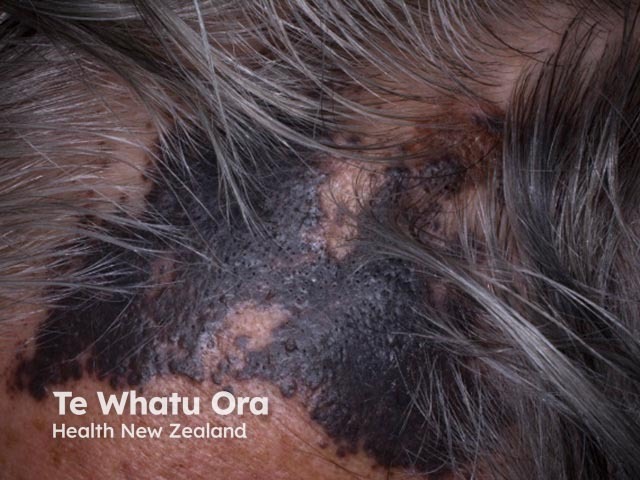
Cutaneous melanoma mestastases
Which melanoma patients benefit from pembrolizumab treatment?
Pembrolizumab is indicated for the treatment of patients with unresectable or metastatic melanoma.
See key clinical-trial evidence about pembrolizumab.
Combination therapy with other immunological drugs and targeted cancer therapy is under investigation and may improve response rates.
How does pembrolizumab work?
The programmed death receptor (PD-1) is a surface molecule expressed on antigen-stimulated T cells as well as monocytes, B cells, and dendritic cells. In normal cells, the PD-1 receptor acts as an immune checkpoint receptor enabling self-tolerance by T cells, thus preventing autoimmune reactions.
When unbound, PD-1 allows the normal response by T-cells to proceed. However, the binding of PD-1 to its ligands, programmed death receptor ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2), suppresses the immune response by inducing downstream signalling that inhibits the proliferation of T cells, cytokine release, and cytotoxicity.
Abnormal PD-L1 expression on the surface of melanoma cells activates PD-1 and suppresses cytotoxic T-cell activity. This T-cell tolerance enables the tumour cells to avoid recognition and attack by the immune system.
Pembrolizumab is a highly selective humanised monoclonal immunoglobulin G4 (IgG4) antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing the PD-1 pathway-mediated inhibition of the immune response against tumour cells.
How is pembrolizumab administered?
- The recommended dose of pembrolizumab is 2 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity.
- Pembrolizumab should be withheld in patients in patients with the following adverse reactions: Grade 2 pneumonitis, Grade 2 or 3 colitis, Grade 2 nephritis, Grade 3 hyperthyroidism, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 3 and up to 5 times the upper limit of normal (ULN), or total bilirubin > 1.5 and up to 3 times the ULN. In addition to withholding this medication, a tapering course of corticosteroids can be initiated.
- Pembrolizumab could be resumed in patients whose adverse reactions recover to Grades 0 or 1.
- The drug should be discontinued permanently in Grade 3 or 4 adverse reactions and when corticosteroid dosing for adverse reactions cannot be reduced below 10 mg/kg/day within 12 weeks.
Potential drug interactions with pembrolizumab
No formal pharmacokinetic drug interaction studies have been conducted with pembrolizumab.
What adverse effects can pembrolizumab cause?
In clinical trials, the most common side effects (≥ 20%) in patients receiving pembrolizumab 2 mg/kg every 3 weeks were:
- Fatigue
- Cough
- Nausea
- Pruritus (itch)
- Rash (unspecified)
- Decreased appetite
- Constipation
- Arthralgia
- Diarrhoea.
The most frequent (≥ 2%) serious adverse drug reactions observed with pembrolizumab were renal failure, dyspnoea (breathlessness), pneumonia, and cellulitis. Pembrolizumab can also cause infusion reactions including anaphylaxis.
Pembrolizumab also has the potential for severe immune-mediated adverse effects. In 411 clinical trial participants with advanced melanoma treated with pembrolizumab, severe immune-mediated adverse effects included pneumonitis, colitis, hypophysitis (inflammation of pituitary gland), hyperthyroidism, hypothyroidism and other endocrinopathies, nephritis, and hepatitis. Other autoimmune diseases have subsequently been reported in association with pembrolizumab.
Cutaneous adverse effects
One report has described cutaneous adverse effects arising in 35 of 83 (42%) patients treated with pembrolizumab. The development of skin problems was associated with longer progression-free intervals. Case reports have described other skin problems during the drug's post-marketing period; these skin problems have included:
- Morbilliform eruption
- Pruritus
- Lichenoid eruption
- Psoriasiform reactions
- Eczema
- Drug-induced vitiligo and poliosis (whitening of hair)
- Stomatitis
- Bullous pemphigoid
- Sarcoid-like granuloma
- Panniculitis
- Stevens–Johnson syndrome / toxic epidermal necrolysis
- Multiple keratoacanthomas
- Eosinophilic fasciitis.
The darkening of hair has been reported in a few patients taking PD-1 inhibitors for non-small cell lung cancer.
See also Cutaneous adverse effects of checkpoint inhibitors.
Psoriasis induced by pembrolizumab
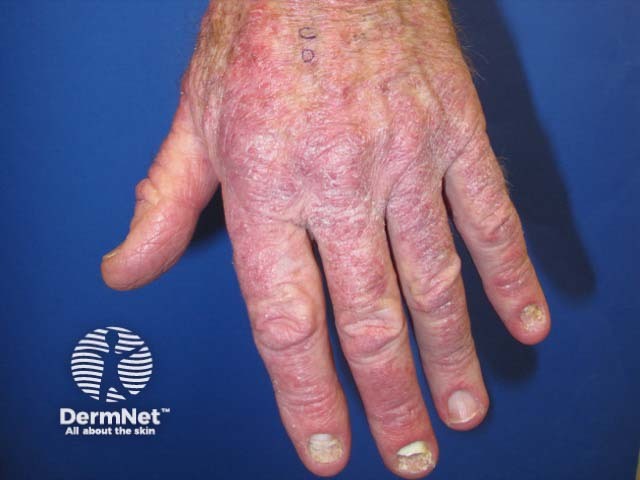
Pembrolizumab-induced psoriasis
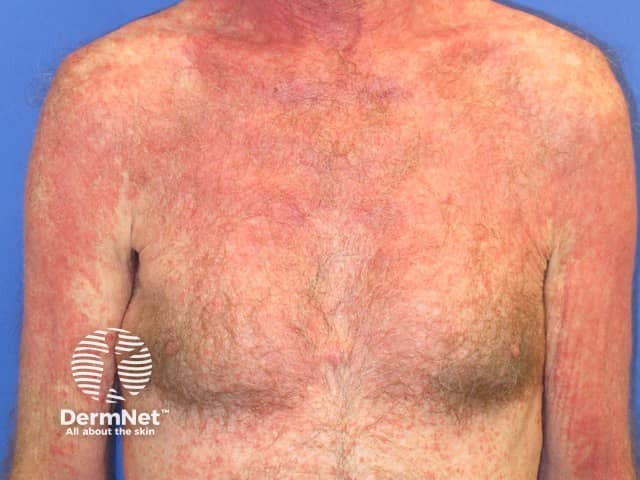
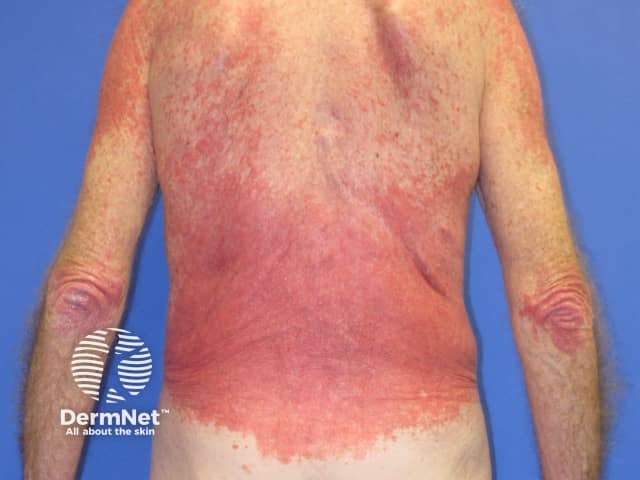
Pembrolizumab-induced psoriasis
Eczema induced by pembrolizumab
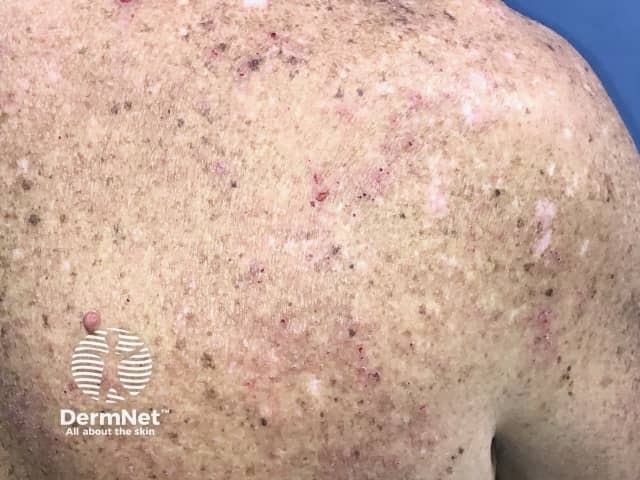
Eczema induced by pembrolizumab
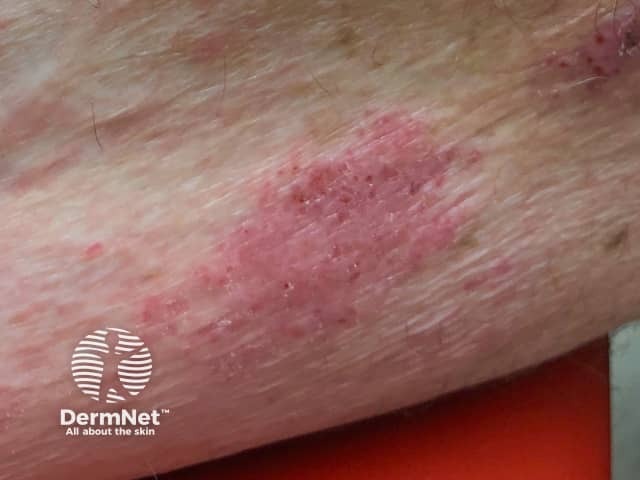
Eczema induced by pembrolizumab
Bullous pemphigoid induced by pembrolizumab
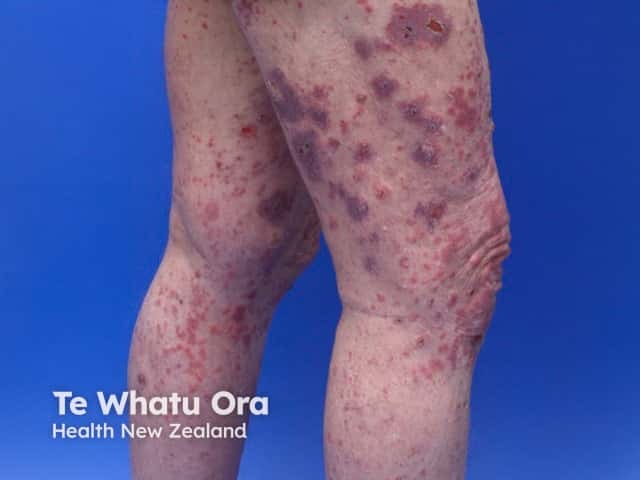
Bullous pemphigoid induced by pembrolizumab
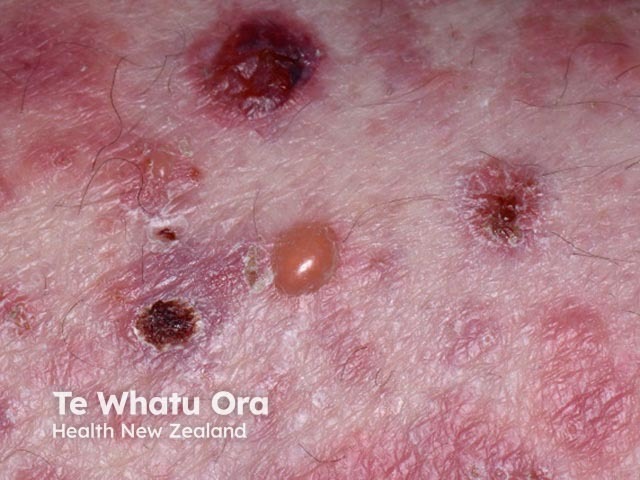
Bullous pemphigoid induced by pembrolizumab
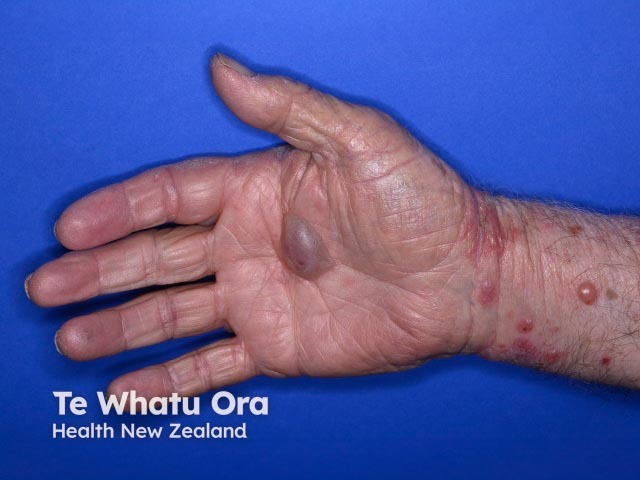
Bullous pemphigoid induced by pembrolizumab
The use of pembrolizumab in particular populations
Pregnant women
- Pembrolizumab may cause fetal harm when administered to a pregnant woman. It is categorised as an FDA Category D drug in pregnancy.
- In animal studies, the potential risks of administering pembrolizumab during pregnancy included increased rates of abortion or stillbirth.
- Human IgG, including IgG4, is known to cross the placenta; therefore, pembrolizumab has the potential to be transmitted from the mother to the developing fetus.
- Based on its mechanism of action, fetal exposure to pembrolizumab may increase the risk of developing immune-mediated disorders or the normal immune response being altered.
Nursing mothers
It is not known whether pembrolizumab or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, mothers should discontinue nursing prior to using pembrolizumab.
Children
The safety and effectiveness of pembrolizumab have not been established in children.
Older people
In one trial of 411 clinical trial patients treated with pembrolizumab, 39% were 65 years and over. No overall differences in safety or efficacy were reported between older patients and younger patients.
Patients with renal impairment
Based on a population pharmacokinetic analysis, no dose adjustment is needed for patients with renal impairment.
Patients with hepatic impairment
Based on a population pharmacokinetic analysis, no dose adjustment is needed for patients with mild hepatic impairment. Pembrolizumab has not been studied in patients with moderate or severe hepatic impairment.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Rivera N, Boada A, Bielsa MI, et al. Hair repigmentation during immunotherapy treatment with an anti–programmed cell death 1 and anti–programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol 2017; 153: 1162–5. DOI: 10.1001/jamadermatol.2017.2106. Journal
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384(9948): 1109-17. DOI: 10.1016/S0140-6736(14)60958-2. PubMed
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369: 134–44. DOI: 10.1056/NEJMoa1305133. PubMed
- Poole RM. Pembrolizumab: first global approval. Drugs 2014; 74: 1973–81. DOI: 10.1007/s40265-014-0314-5. PubMed
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 2015; 151: 1206–12. DOI: 10.1001/jamadermatol.2015.1916. PubMed
- Freites-Martinez A, Kwong BY, Rieger KE, Coit DG, Colevas AD, Lacouture ME. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol 2017; 153: 694–7. DOI: 10.1001/jamadermatol.2017.0989. PubMed Central
- Chan KK, Magro C, Shoushtari A, et al. Eosinophilic fasciitis following checkpoint inhibitor therapy: four cases and a review of literature. Oncologist. 2020;25(2):140–9. doi:10.1634/theoncologist.2019-0508. PubMed
- FDA approves pembrolizumab for cutaneous squamous cell carcinoma. US Food & Drug Administration.
On DermNet
- Melanoma
- Metastatic melanoma
- Ipilimumab
- Vemurafenib
- Dabrafenib
- Drug-induced vitiligo
- Key clinical-trial evidence for pembrolizumab
- Adjuvant therapies for melanoma
- Nivolumab
- Targeted cancer therapies
- Cutaneous adverse effects of anti-melanoma therapies
- Cutaneous adverse effects of checkpoint inhibitors
- Adverse cutaneous reactions to drugs
Other websites
- KEYTRUDA® (pembrolizumab) for injection, for intravenous use — US FDA prescribing information, September 2014 (pdf)
- Pembrolizumab for treating advanced melanoma after disease progression with ipilimumab — UK National Institute for Health and Care Excellence technology appraisal guidance. Updated September 2017
- Pembrolizumab for advanced melanoma not previously treated with ipilimumab — UK National Institute for Health and Care Excellence technology appraisal guidance, updated September 2017
- Keytruda — Medsafe New Zealand manufacturer datasheet (pdf)
- Cancer treatment with Keytruda — Information for patients in New Zealand provided by MSD
- Keytruda® (pembrolizumab) Healthcare Professional Portal — MSD Oncology
