Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Vemurafenib — extra information
Treatments Lesions (cancerous)
Vemurafenib
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet Editor in Chief: Adjunct A/Prof Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand. Updated March 2018.
Introduction
How it works
Demographics
Detection of BRAF-600 mutation
Key clinical-trial evidence
Patients with BRAF V600E mutation-positive melanoma with brain metastases
Cobimetinib combined with vemurafenib in advanced BRAF (V600)-mutant melanoma (coBRIM)
Dosage and administration
Adverse events
Cutaneous adverse reactions
Warnings and precautions
Drug interactions
Use
Future considerations
Introduction
Vemurafenib (ZELBORAF®) is a kinase inhibitor indicated for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation as detected by an FDA-approved test.
In August 2011, the US Food and Drug Administration (FDA) approved vemurafenib for the first-line treatment of both metastatic and unresectable (inoperable) melanoma. Vemurafenib was registered by MedSafe for use in New Zealand in February 2012 but is not currently funded by PHARMAC (August 2018). It can also be taken in combination with cobimetinib, a MEK inhibitor.
Vemurafenib and vemurafenib with cobimetinib have demonstrated prolonged survival in advanced melanoma patients.
How does vemurafenib work?
Vemurafenib is a threonine kinase inhibitor, one of a new class of medicines known as epidermal growth factor receptor (EGRF) inhibitors or targeted therapy.
- Vemurafenib blocks a critical protein molecule called BRAF, which is mutated (changed) in up to 50% of patients with melanoma.
- BRAF is a protein that is part of the cell signalling pathway that controls cell growth in a number of different tissues in the body.
- Mutations that lock the BRAF protein in an active state can cause excessive signalling in the pathway, leading to uncontrolled growth of melanocytes (pigment cells).
- When the activity of mutant BRAF is blocked, cancer cells stop growing and die.
Which patients benefit from vemurafenib treatment?
- Vemurafenib is specifically indicated for patients with melanoma whose tumours express a gene mutation called BRAF V600.
- The BRAF protein produced as a result of this gene mutation has the amino acid (building blocks of protein) glutamate instead of the amino acid valine at position 600.
- Vemurafenib is not indicated for use in patients without the V600 mutation.
How are BRAF-600 mutations detected?
Melanoma tumours that carry the BRAF V600 mutation can be identified by a companion diagnostic test developed by the pharmaceutical company Roche, USA, and approved by the FDA.
The test is called the cobas® 4800 BRAF V600 Mutation Test and must be performed on melanoma biopsy samples from all patients before starting treatment with vemurafenib.
The test has several advantages including:
- Use of formalin-fixed paraffin-embedded samples (ie, existing biopsy tissue)
- Sensitivity and reliability for detecting BRAF V600 mutations
- Gives quick results, allowing doctors to know whether a person with metastatic melanoma is eligible for treatment with vemurafenib.
Key clinical-trial evidence about vemurafenib
The FDA approval of vemurafenib was based on results from two clinical studies (BRIM3 and BRIM2) in patients with BRAF V600E mutation-positive, inoperable or metastatic melanoma as determined by the cobas® BRAF Mutation Test.
BRIM3
BRIM3 (B-RAF Inhibitor in Melanoma phase 3) was a global, randomised, open-label, multicenter, advanced (phase III) study that compared 960 mg of vemurafenib given orally twice daily with dacarbazine (previous standard of care for advanced melanoma treatment) 1000 mg/m2 given IV on day 1, every 3 weeks in 675 patients with previously untreated BRAF V600E mutation-positive, unresectable (inoperable) or metastatic melanoma. Treatment continued until disease progression, unacceptable toxicity, and/or consent withdrawal.
Key results are tabulated below:
Vemurafenib (No. of patients = 337) |
Dacarbazine (No. of patients = 338) |
|
|---|---|---|
Overall survival (OS) |
||
Number of Deaths |
78 (23%) |
121 (36%) |
Median OS (months) |
13.6 |
10.3 |
Median Follow-up (months) |
13.4 |
9.2 |
Progression-free survival (PFS) |
||
Median PFS (months) |
5.3 |
1.6 |
Complete tumour shrinkage (% patients) |
1% |
0 |
Partial tumour shrinkage (% patients) |
47.4% |
5.5% |
- Improvement in OS, PFS and tumour shrinkage with vemurafenib was seen in patients regardless of age, sex, or disease risk factor.
- Kaplan–Meier estimates of OS rates for vemurafenib versus dacarbazine were 56% versus 46%, 30% versus 24%, 21% versus 19% and 17% versus 16% at 1, 2, 3 and 4 years, respectively.
BRIM2
BRIM2 was a global, single-arm, multicentre, open-label early-phase (phase II) study that enrolled 132 patients with previously treated BRAF V600 mutation-positive metastatic melanoma. The primary endpoint of the study was the best overall response rate.
Data showed that:
- 53 per cent of patients treated with vemurafenib had tumour shrinkage.
- Three patients (2.3%) showed complete tumour shrinkage and 66 (50.0%) showed partial tumour shrinkage.
- Patients who participated in BRIM2 lived a median of 6.7 months without their disease getting worse (median PFS).
- Median OS has not yet been reached after a median follow-up of 10 months.
Patients with BRAF V600E mutation-positive melanoma with brain metastases
- This was an open-label multicentre, single-arm 2 cohort trial in patients with BRAF V600 mutation-positive melanoma with brain metastases.
- Patients received 960 mg vemurafenib orally twice daily until disease progression or unacceptable toxicity and were followed up for 24 months.
- Patients in cohort A had received no prior local therapy for brain metastasis but those in cohort B had received at least one prior local therapy (surgery or radiation).
- The primary efficacy outcome measure was the confirmed best overall response rate in the brain in Cohort A, as assessed by an independent radiology review committee using Response Evaluation Criteria in Solid Tumors (RECIST v1.1).
Key results are tabulated below.
% Patients |
Cohort A (N = 90) |
Cohort B (N = 56) |
|
|---|---|---|---|
Confirmed best overall response rate in brain |
18% |
18% |
|
Complete response |
2% |
0% |
|
Partial response |
16% |
18% |
|
The median duration of response, months (95%CI; Kaplan Meier estimate) |
4.6 (2.9, 6.2) |
6.6 (2.8, 10.7) |
|
Cobimetinib combined with vemurafenib in advanced BRAF (V600)-mutant melanoma (coBRIM)
- 495 adult patients with histologically confirmed BRAF(V600) mutation-positive unresectable stage IIIC or stage IV melanoma were randomly assigned to the cobimetinib plus vemurafenib group (n=247) or to the placebo plus vemurafenib group (n=248).
- At a median follow-up of 14·2 months, the investigator-assessed median progression-free survival was 12·3 months (95% CI 9·5–13·4) for cobimetinib and vemurafenib versus 7·2 months (5·6–7·5) for placebo and vemurafenib (HR 0·58 [95% CI 0·46-0·72], p<0·0001).
- The median overall survival was 22·3 months (95% CI 20·3-not estimable) for cobimetinib and vemurafenib versus 17·4 months (95% CI 15·0–19·8) for placebo and vemurafenib (HR 0·70, 95% CI 0·55–0·90; p=0·005).
- The safety profile for cobimetinib and vemurafenib was tolerable and manageable.
- The most common grade 3–4 adverse events occurring at a higher frequency in patients in the cobimetinib and vemurafenib group compared with the vemurafenib group were γ-glutamyl transferase increase (36 [15%] in the cobimetinib and vemurafenib group vs 25 [10%] in the placebo and vemurafenib group), blood creatine phosphokinase increase (30 [12%] vs one [< 1%]), and alanine transaminase increase (28 [11%] vs 15 [6%]).
- Pyrexia (six patients [2%]) and dehydration (five patients [2%]) were the most common serious adverse events reported in the cobimetinib and vemurafenib group.
- These data confirm the clinical benefit of cobimetinib combined with vemurafenib and support the use of the combination as a standard first-line approach to improve survival in patients with advanced BRAF(V600)-mutant melanoma.
- The addition of cobimetinib to vemurafenib was associated with a significant improvement in progression-free survival among patients with BRAF V600-mutated metastatic melanoma, at the cost of some increase in toxicity.
Patients with wild-type BRAF melanoma
Vemurafenib has not been studied in patients with wild-type BRAF melanoma.
Dosage and administration of vemurafenib
The presence of BRAF V600E mutation in tumour specimens should be confirmed prior to initiation of treatment with vemurafenib.
- Each tablet contains 240 mg of vemurafenib.
- Recommended dose: 960 mg orally twice daily, approximately 12 hours apart with or without a meal.
- The tablets should be swallowed whole with a glass of water and should not be chewed or crushed.
- If a dose is missed, it can be taken up to 4 hours prior to the next dose to maintain the twice-daily regimen. Both doses should not be taken at the same time.
- Patients are treated with vemurafenib until disease progression or unacceptable toxicity occurs.
Adverse events due to vemurafenib
There are only limited data about the safety and efficacy of vemurafenib due to small patient numbers treated up to now. Treatment should be monitored including monthly liver function tests and as clinically indicated.
The most common adverse reactions (≥ 10% treated patients) reported with vemurafenib in BRIM3 and BRIM2 trials were:
- Arthralgia
- Rash
- Alopecia (hair loss)
- Fatigue
- Photosensitivity reactions
- Nausea
- Pruritus
- Skin papilloma
- Headache
- Dry skin.
Management of symptomatic adverse drug reactions may require dose reduction, treatment interruption, or treatment discontinuation. Dose reductions resulting in a dose below 480 mg twice daily are not recommended.
Cutaneous adverse reactions from vemurafenib
Some of the cutaneous side effects of vemurafenib are similar to those described with other EGFR and protein kinase inhibitors, but cutaneous squamous cell carcinoma (SCC) appears to be a specific problem. Concern has been expressed that vemurafenib may also lead to more melanocytic lesions, including common moles and perhaps new primary melanoma. The reactions are dose-related and may settle down even with continued therapy. Cutaneous side effects include:
- Toxic erythema (morbilliform or scarlatiniform drug rash)
- Drug hypersensitivity syndrome (DRESS)
- Photosensitivity, including rapid-onset extreme sunburn on minimal exposure to the sun or other sources of UVA even through window glass
- Scaly skin lesions, ranging from papillomas to keratoacanthoma and other types of cutaneous squamous cell carcinoma
- Acute neutrophilic dermatosis (Sweet syndrome)
- Panniculitis, resulting in painful red nodules
- Folliculocentric rash, red dry bumps corresponding with hair follicles, including keratosis pilaris
- Painful, red, scaly palms and soles
- Dermatitis in areas exposed to radiation (radiation-recall dermatitis)
- Hair loss on scalp and body (see alopecia from drugs)
- Acne-like eruptions and milia
- Drug-induced vitiligo
- Transient acantholytic dermatosis with monotherapy
- Involution of melanocytic naevi (moles), particularly papillomatous, dermatoscopic globular-pattern moles; and; enlargement of other flat, mainly dermatoscopic reticular-pattern naevi
- New primary melanoma has been reported.
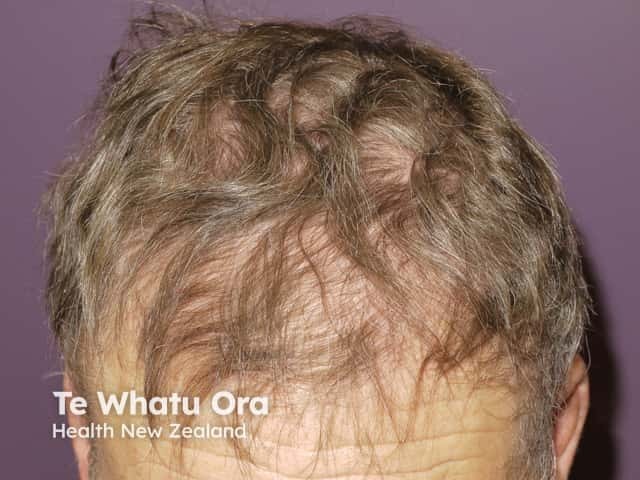
Alopecia

Dry skin
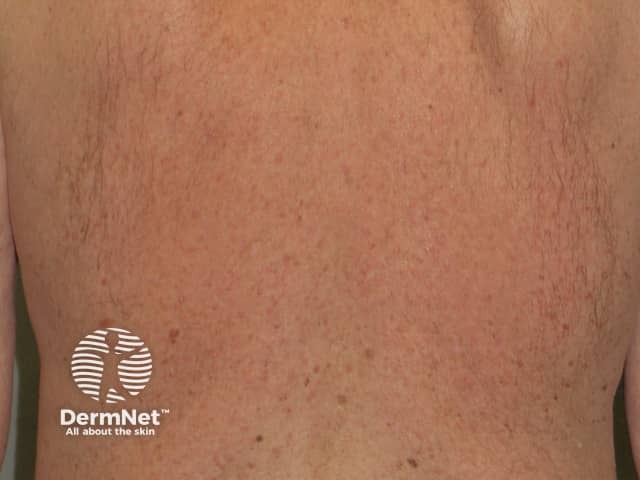
Nonspecific rash
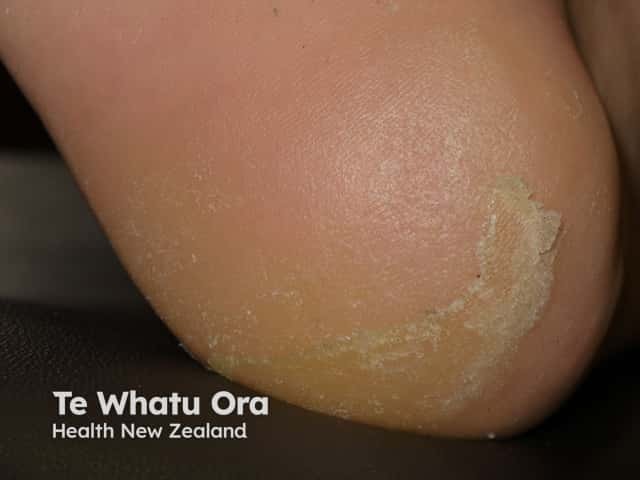
Keratoderma
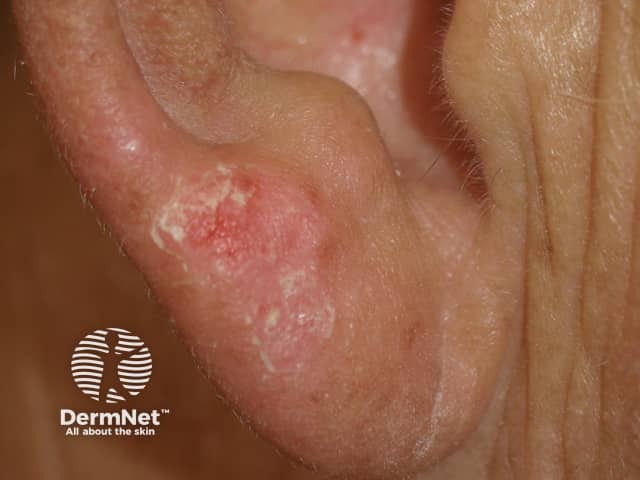
Photosensitivity
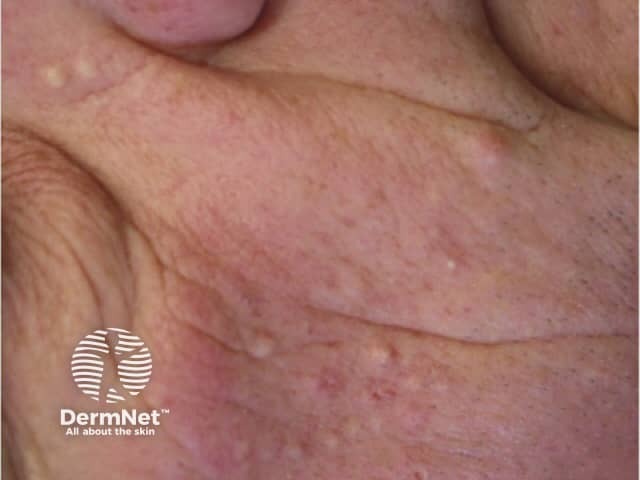
Folliculitis
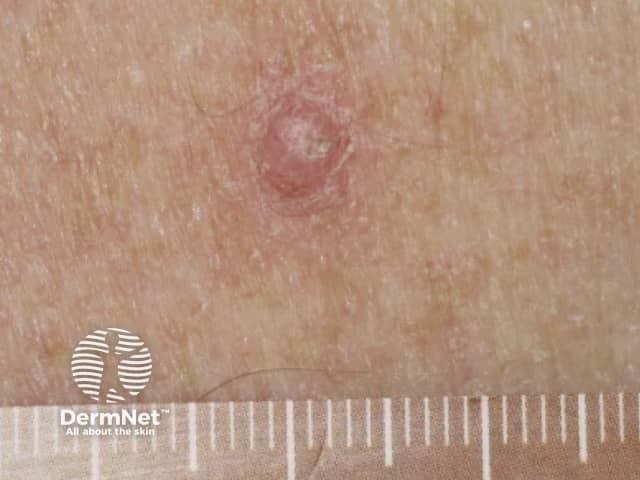
Papilloma
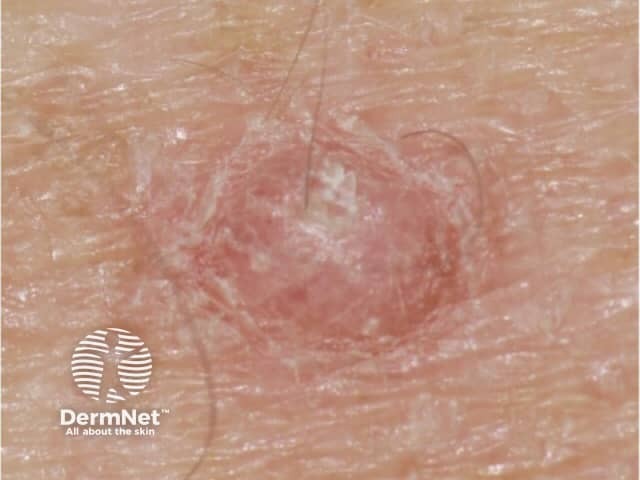
Close-up papilloma
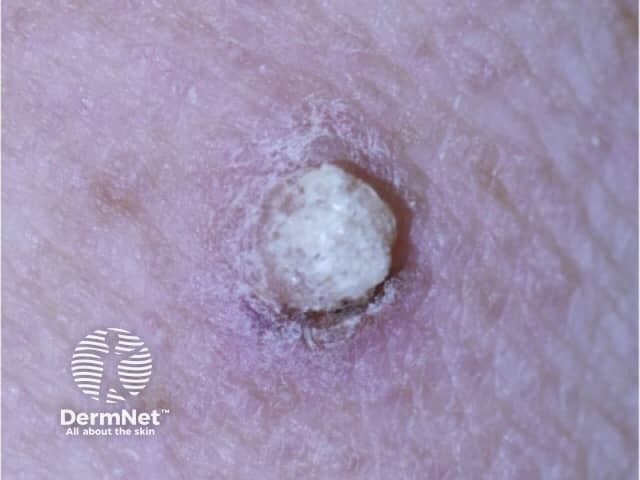
Papilloma
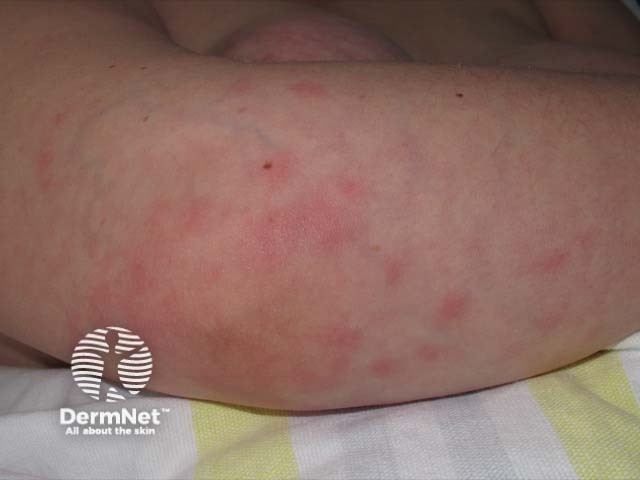
Sweet syndrome
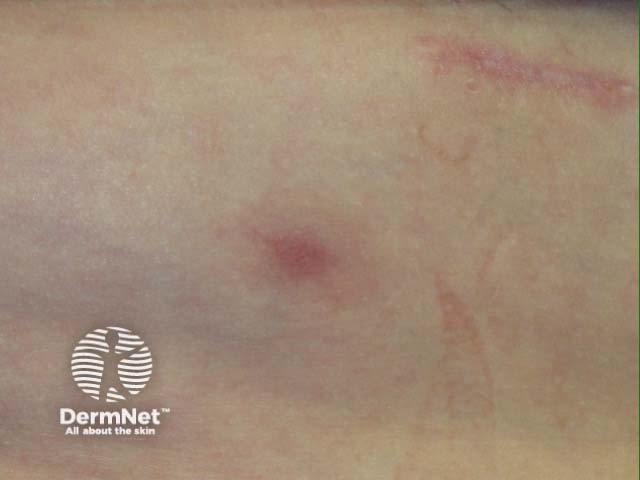
Panniculitis
Warnings and precautions
- QT Prolongation — ECG and electrolytes should be monitored before and during treatment. Correct electrolyte abnormalities and control for cardiac risk factors for QT prolongation; vemurafenib should be withheld for QTc of 500 ms or greater.
- Hepatotoxicity — liver enzymes and bilirubin levels should be measured before initiating vemurafenib and monitored monthly thereafter.
- Photosensitivity — advise patients to avoid sun exposure.
- Serious ophthalmologic reactions — monitor patients for signs and symptoms of uveitis.
- Renal failure — serum creatinine should be measured before initiating vemurafenib and levels monitored periodically during treatment.
Drug interactions with vemurafenib
- Vemurafenib levels can be altered when administered together with CYP3A4 (a liver enzyme involved in drug metabolism) inhibitors (eg, ketoconazole, itraconazole, clarithromycin, atazanavir, nefazodone, saquinavir, telithromycin, ritonavir, indinavir, nelfinavir, voriconazole) and inducers (eg, phenytoin, carbamazepine, rifampicin, rifabutin, rifapentine, phenobarbital).
- If coadministration cannot be avoided, consider a dose reduction of the concomitant drug.
- Vemurafenib may increase exposure when administered together with warfarin.
- When vemurafenib is used together with warfarin, consider additional INR (international normalised ratio – a measurement of blood coagulation) monitoring.
Use in specific populations
- Nursing mothers — discontinue nursing when receiving vemurafenib.
- Paediatric use — safety and efficacy in patients below the age of 18 have not been established.
- Geriatric use — in clinical trials, the overall survival, progression-free survival and best overall response rate were similar in the elderly as compared with younger patients.
- Hepatic impairment — no adjustment to the starting dose is needed for patients with pre-existing mild and moderate hepatic impairment.
- Renal impairment — no adjustment to the starting dose is needed for patients with pre-existing mild and moderate renal impairment.
Future considerations for vemurafenib
- Research trials are on-going.
- One of the challenges of BRAF inhibition is that the responses, while dramatic and rapid in onset, last on average only approximately 6–7 months.
- Resistance quickly develops and this is now an active area of investigation.
- Combinations of vemurafenib with other medications, such as the MAPK signalling pathway inhibitor trametenib, may prove useful in melanoma patients.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–16. PubMed
- Russo AE, Torrisi E, Bevelacqua Y, Perrotta R, Libra M, McCubrey JA, Spandidos DA, Stivala F, Malaponte G. Melanoma: molecular pathogenesis and emerging target therapies (Review). Int J Oncol 2009; 34: 1481–9. PubMed
- Ribas A, Kim KB, Schuchter LM, Gonzalez R, Pavlick AC, et al. BRIM-2: An open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAF V600E mutation-positive metastatic melanoma. J Clin Oncol 2011; 29: 8509. Journal
- Singh AG, Tchanque-Fossuo CN, Elwood H, Durkin JR. BRAF inhibitor and hairy cell leukemia-related transient acantholytic dermatosis. Dermatol Online J. 2020;26(2):13030/qt3ps33564. Journal
- Sinha R, Edmonds K, Newton-Bishop JA, et al. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: practical advice on diagnosis, prevention and management of the main treatment-related skin toxicities. Br J Dermatol 2012: 167; 987–94. PubMed
- Haenssle HA, Kraus SL, Brehmer F, Kretschmer L, Völker B, Asper H, Kapp A, Gutzmer R. Dynamic changes in nevi of a patient with melanoma treated with vemurafenib: importance of sequential dermoscopy. Arch Dermatol 2012; 148: 1183–5. PubMed
- Belum VR, Fischer A, Choi JN, Lacouture ME. Dermatological adverse events from BRAF inhibitors: a growing problem. Curr Oncol Rep 2013; 15: 249–59. PubMed
- Ascierto PA, McArthur GA, Dréno B, Atkinson V, Liszkay G, Di Giacomo AM, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 2016; 17: 1248–60. PubMed
- Chapman PB, Robert C, Larkin J, Haanen JB, Ribas A, et al. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: final overall survival results of the randomized BRIM-3 study. Ann Oncol 2017; 28: 2581–87. PubMed
- Brigitte Dreno, Paolo A Ascierto, Victoria Atkinson, Gabriella Liszkay, Michele Maio et al. Health-related quality of life impact of cobimetinib in combination with vemurafenib in patients with advanced or metastatic BRAFV600 mutation–positive melanoma. Br J Cancer 2018; 118: 777–84. doi: 10.1038/bjc.2017.488. PubMed
- Blank CU, Larkin J2, Arance AM, Hauschild A, Queirolo P, et al. Open-label, multicentre safety study of vemurafenib in 3219 patients with BRAFV600 mutation-positive metastatic melanoma: 2-year follow-up data and long-term responders' analysis. Eur J Cancer 2017; 79: 176–84. PubMed
On DermNet
- Melanoma
- Metastatic melanoma
- Cobimetinib
- Ipilimumab
- Dabrafenib
- Trametenib
- Genetics of melanoma
- Drug-induced vitiligo
- Targeted therapy
- Adjuvant therapies for melanoma
Other websites
- Vemurafenib — Melanoma New Zealand
- Zelboraf prescribing information — DailyMed
- 'Time to Celebrate'; New Metastatic Melanoma Agent Wows ASCO — Medscape Medical News from 2011 Annual Meeting of the American Society of Oncology®
- Vemurafenib for treating locally advanced or metastatic BRAF V600 mutation‑positive malignant melanoma — NICE technology appraisal guidance, January 2015
- Zelboraf prescribing information — Genentech Inc. USA
