Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Adjuvant therapies for melanoma — extra information
Treatments Lesions (cancerous)
Adjuvant therapies for melanoma
Author: Matthew Howard, Clinic/Research Fellow, Victorian Melanoma Service, Melbourne, VIC, Australia. DermNet Editor in Chief: Adjunct A/Prof. Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. November 2019.
Introduction
Demographics
Introduction
Contraindications
Introduction - neoadjuvant therapy
Benefits
Disadvantages
Side effects and risks
What is adjuvant therapy?
Adjuvant therapy is defined as a treatment given after the initial therapy. In the case of cutaneous melanoma, adjuvant therapy is a form of treatment considered after complete surgical excision of the melanoma (the first-line treatment) [1].
Adjuvant immunotherapy aims to eliminate residual microscopic melanoma cells via the immune system, to reduce the risk of future cancer recurrence, and to improve the overall chance of cure [1].
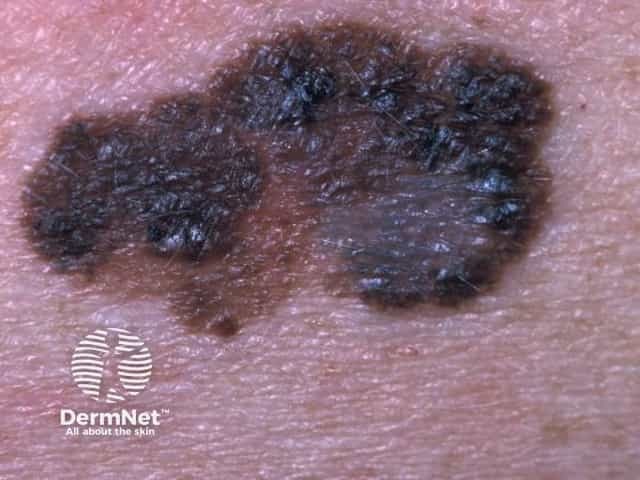
Superficial spreading melanoma
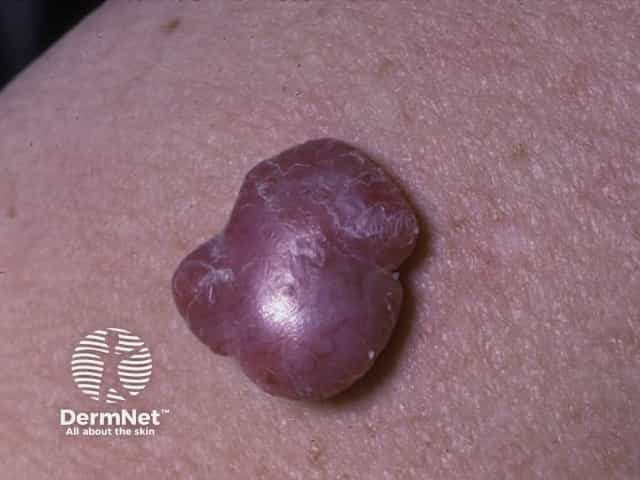
Nodular melanoma
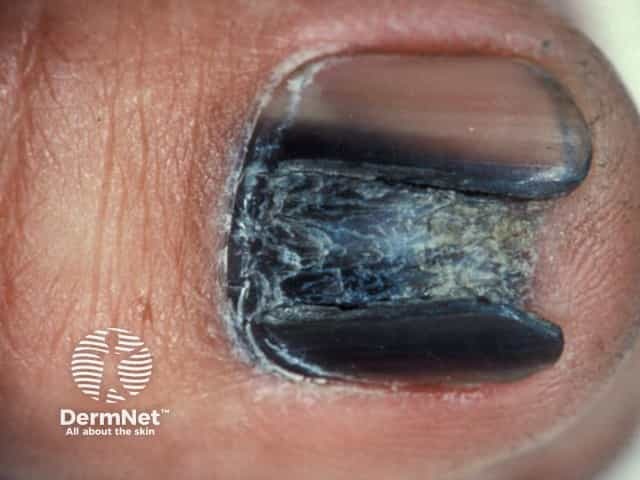
Acral melanoma
Who uses adjuvant therapies?
Indications for consideration of adjuvant therapies for cutaneous melanoma include [1–3]:
- Unfavourable localised disease — intermediate-thickness melanoma (Breslow thickness > 1 mm) with or without additional pathological prognostic factors such as ulceration or increased mitotic rate
- Stage III metastatic melanoma (this is when there is histological evidence of melanoma in regional lymph node/s, in transit, or satellite metastasis)
- Localised melanoma at high risk for local recurrence due to inadequate excision margins or perineural invasion (also termed neurotropism).
What are adjuvant therapies for melanoma?
Immunotherapy and targeted cancer therapy against driver mutations have proven a benefit in stage IV metastatic melanoma (when distant metastases have been detected). Adjuvant therapy is also reported to reduce the relative risk of recurrence by 40% in stage III metastatic melanoma with a 15–25% absolute reduction in local and distant recurrence risk. Overall survival data are not yet reported for immunotherapy, but significant overall survival benefits are seen with adjuvant-combined dabrafenib and trametinib in BRAF–mutant stage III metastatic melanoma.
The results of adjuvant treatment have been published using immunotherapy, targeted therapy, or radiotherapy that has been given for up to a total of 12 months.
Immunotherapy
There are two classes of immune checkpoint inhibitors, as of 2019.
- The cytotoxic T-lymphocyte-associated protein 4 (CTLA4) antibody, ipilimumab (Yervoy®) [4].
- Programmed cell death protein 1 (PD1) antibodies:
- Nivolumab (Opdivo®) [4]
- Pembrolizumab (Keytruda®) [5].
Immunotherapies are given by intravenous infusion every three weeks [4].
Targeted therapies
Targeted therapies block the mitogen-activated protein (MAP) kinase pathway [6].
- BRAF inhibitors include dabrafenib, vemurafenib, and encorafenib.
- MEK inhibitors include trametinib, cobimetinib, and binimetinib.
BRAF inhibitors are administered orally twice daily; a MEK inhibitor is usually taken orally once daily [8]. A BRAF and MEK inhibitor are usually used together in combination therapy to limit early tumour resistance to single-agent therapy [7].
Radiation therapy
Stereotactic external beam radiotherapy can be considered after therapeutic lymphadenectomy to reduce the rate of nodal recurrence, particularly when there is an extranodal extension of metastatic melanoma [8,9].
What are the contraindications with adjuvant therapies?
Typically, patients with lower risk or thin localised melanoma are not currently offered adjuvant therapies (Stage I/IIA disease) [1,2].
The relative contraindications for the use of immunotherapy include:
- Pregnancy — due to concerns about the potential loss of maternal immunological tolerance to a fetus [10]
- A history of poorly controlled or uncontrolled autoimmune disease — activation of the immune system by immunotherapy might lead to a worsening of the autoimmune condition [11]
- Organ transplantation — there have been isolated reports of rapid rejection of the transplanted organ when checkpoint inhibitors have been used. Immunotherapy may still be considered in renal transplant patients if they are also dialysis candidates [12]
- A serious or life-threatening adverse reaction to previous immunotherapy [13].
Targeted therapy using BRAF inhibitors is contraindicated for patients without the BRAF V600 driver mutation [7].
What is neoadjuvant therapy?
Unlike adjuvant therapy, which is given after surgery to remove melanoma, neoadjuvant therapy is an induction treatment commenced before the excision. It is typically used when the primary melanoma is unresectable (ie, it is a large tumour, the excision defect can’t be closed, or the excision would be unacceptable cosmetically) or when any nodal or visceral melanoma metastases are too bulky to safely remove. The intention of neoadjuvant therapy is to reduce the size of the tumour to allow the surgery to take place [14].
What are the benefits of adjuvant therapy for stage III metastatic melanoma?
Early results of clinical research programmes are demonstrating consistent, clinically meaningful benefits from targeted therapies and immunotherapies in stage III metastatic melanoma when the melanoma has been completely resected.
Immunotherapy
Initial research data on immunotherapy using ipilimumab in stage III metastatic melanoma is from two major studies. The chance of overall survival at 5 years was improved by 28%, and the risk of metastatic melanoma recurrence at 5 years was improved by 24% in those randomised to ipilimumab compared to placebo [15].
- In the CheckMate 238 trial, the recurrence-free survival was reduced by 35% in those randomised to receive nivolumab compared to ipilimumab with reduced serious adverse events [4].
- In the KEYNOTE-054 study, patients randomised to pembrolizumab had a 43% reduction in risk of recurrence or death compared to placebo [5].
Targeted therapy
Studies into BRAF inhibitors and BRAF/MEK dual inhibition for patients with stage III metastatic melanoma were conducted prior to the immunotherapy studies. Key results are listed below. A consistent improvement in the overall survival (OS) of patients was demonstrated with the use of single agent MEK/BRAF inhibition compared to placebo. This benefit was increased with dual inhibition [7,16]. Due to the adverse effects of targeted therapy, one of the components may need to be stopped [17].
Inhibitor |
CR + PR% |
Median PFS, Median OS |
|---|---|---|
Vemurafenib [18] |
53% |
6.9 months, 13.6 months |
Dabrafenib [19] |
50% |
6.7 months, 18 months |
Trametinib [20] |
22% |
4.8 months (PFS only) |
Dabrafenib + trametinib [7] |
67% |
11 months, 25.1 months |
Dabrafenib + trametinib versus vemurafenib [16] |
64% |
12.6 months, 25.6 months |
CR: complete response; PR: partial response; CR + PR%: percentage of patients with a partial or complete response; PFS: progression-free survival; OS: overall survival
Adjuvant radiotherapy
Local control of melanoma may be enhanced by adjuvant radiotherapy if the risk of local recurrence remains unacceptably high after surgical excision such as with:
- Inadequate wide excision margins, often due to anatomical or cosmetic constraints around the eye/conjunctiva, nose, lips, or ears [21]
- Satellite lesions
- Neurotropism (perineural invasion) on histology [8,9]
- Desmoplastic melanoma, due to its tendency for local recurrence.
Although randomised controlled trials have not evaluated the benefit of adjuvant radiotherapy, several large observational cohorts have reported positive results with 50–85% improvement in risk of local recurrence. Possible evidence was also observed for decreased nodal recurrence [22–24].
The local inflammatory effects resulting from radiation therapy may provide a synergistic boost to the efficacy of immunotherapy [25,26]. Further research is underway to evaluate its benefits [27,28].
What are the disadvantages of adjuvant therapies?
The comparative benefits of immunotherapy to traditional chemotherapy have been game-changing for the treatment of metastatic melanoma in some patients. However, in many patients, melanoma does not respond to immunotherapy or only partially responds. In addition, targeted therapies are only indicated for those whose melanoma carries the BRAF V600 driver mutation (approximately 40–50% of cutaneous melanoma) [29].
There are questions still to be answered about adjuvant therapy for melanoma.
- Does adjuvant immunotherapy improve overall survival for patients with stage III metastatic melanoma?
- Is it better to give adjuvant immunotherapy for stage III metastatic melanoma or wait until stage IV metastatic melanoma disease has developed?
- If a patient has a BRAF V600 mutation, should we use targeted therapy or immunotherapy first?
- Would adjuvant immunotherapy or targeted therapy reduce the risk of the disease if used at an earlier stage of melanoma? A study is currently recruiting participants to answer this question in stage IIB-C melanoma (2019).
What are the side effects and risks of adjuvant therapies?
Adjuvant treatments are generally well tolerated with comparative greater short-term toxicity with BRAF/MEK combined inhibition compared to immunotherapy; however, there is a risk of permanent toxicity with adjuvant immunotherapy.
Side effects of immunotherapy
Immunotherapy can result in side effects due to immune activation [13,30]. The rates described below are for PD1 inhibitors (nivolumab and pembrolizumab); immune-related adverse events are typically more frequent with CTLA-4 inhibition (ipilimumab).
- Fatigue ~25% on a PD1 inhibitor
- Skin: pruritus, dermatitis, or vitiligo in ~34%
- Gastrointestinal tract: colitis causing diarrhoea in 13% (the risk is greater with the combination of ipilimumab and nivolumab)
- Thyroid dysfunction: hyperthyroidism or hypothyroidism in ~10%
- Arthralgia ~7%
- Hepatitis 5–10%
- Pneumonitis 2–4%
- Myositis
- Acute kidney injury
- Rarely, hypophysitis.
See also Cutaneous adverse effects of checkpoint inhibitors.
Side effects of targeted therapies
Combined dabrafenib/trametinib causes the following adverse effects [17].
- Pyrexia
- Fatigue
- Nausea
- Headache
- Diarrhoea
- Vomiting
- Arthralgia
- Dermatitis
- New cutaneous skin cancer, especially squamous cell carcinoma (a BRAF inhibitor effect)
- Cardiac effects (left ventricular dysfunction)
- Ocular effects (blurred vision, chorioretinopathy)
- Pulmonary effects (interstitial lung disease, pneumonitis).
Side effects of radiotherapy
Acute side effects of radiotherapy may include [31,32]:
- Desquamation
- Erythema
- Organ-related inflammation and oedema (eg, oesophagitis, mucositis, pneumonitis)
- Fatigue.
Late side effects of radiotherapy may include:
- Hypopigmentation or hyperpigmentation
- Telangiectasia
- Epidermal atrophy and fragility
- Alopecia and sweat gland atrophy
- Necrosis of soft tissue, cartilage, or bone
- Radiation-induced malignancy — squamous cell carcinoma, basal cell carcinoma.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Napolitano S, Brancaccio G, Argenziano G, et al. It is finally time for adjuvant therapy in melanoma. Cancer Treat Rev. 2018 Sep;69:101–111. doi: 10.1016/j.ctrv.2018.06.003. Epub 2018 Jun 9. PMID: 29957365. PubMed
- Moreno Nogueira JA, Valero Arbizu M, Pérez Temprano R. Adjuvant treatment of melanoma. ISRN Dermatol. 2013;2013:545631. doi: 10.1155/2013/545631. Epub 2013 Feb 17. PMID: 23476798; PMCID: PMC3588212. PubMed Central
- Gershenwald JE, Scolyer RA, Hess KR, et al; for members of the American Joint Committee on Cancer Melanoma Expert Panel and the International Melanoma Database and Discovery Platform. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017 Nov;67(6):472–92. doi: 10.3322/caac.21409. Epub 2017 Oct 13. PMID: 29028110; PMCID: PMC5978683. PubMed
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV Melanoma. N Engl J Med. 2017 Nov 9;377(19):1824–35. doi: 10.1056/NEJMoa1709030. Epub 2017 Sep 10. PMID: 28891423. Journal
- Eggermont A, Blank C, Mandala M, et al. Adjuvant Pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018 May 10;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. Epub 2018 Apr 15. PMID: 29658430. PubMed
- Australian Medicines Handbook. Adelaide: Australian Medicines Handbook Pty Ltd; 2018.
- Long G, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014 Nov 13;371(20):1877–88. doi: 10.1056/NEJMoa1406037. Epub 2014 Sep 29. PMID: 25265492. PubMed
- Ballo M, Ang K. Radiotherapy for cutaneous malignant melanoma: rationale and indications. Oncology (Williston Park). 2004 Jan;18(1):99–107; discussion 107-10, 113-4. PMID: 14768409. PubMed
- Rao NG, Yu HH, Trotti A 3rd, Sondak VK. The role of radiation therapy in the management of cutaneous melanoma. Surg Oncol Clin N Am. 2011 Jan;20(1):115–31. doi: 10.1016/j.soc.2010.09.005. PMID: 21111962. PubMed
- Poulet FM, Wolf JJ, Herzyk DJ, DeGeorge JJ. An evaluation of the impact of PD-1 pathway blockade on reproductive safety of therapeutic PD-1 inhibitors. Birth Defects Res B Dev Reprod Toxicol. 2016 Apr;107(2):108–19. doi: 10.1002/bdrb.21176. Epub 2016 Apr 7. PMID: 27062127. PubMed
- Abdel-Wahab N, Shah M, Lopez-Olivo M, Suarez-Almazor M. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med. 2018 Jan 16;168(2):121–30. doi: 10.7326/M17-2073. Epub 2018 Jan 2. PMID: 29297009. PubMed
- Kittai A, Oldham H, Cetnar J, Taylor M. Immune checkpoint inhibitors in organ transplant patients. J Immunother. 2017 Sep;40(7):277–81. doi: 10.1097/CJI.0000000000000180. PMID: 28719552. PubMed
- Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017 Jul 1;28(suppl_4):iv119-iv142. doi: 10.1093/annonc/mdx225. Erratum in: Ann Oncol. 2018 Oct 1;29(Suppl 4):iv264-iv266. Erratum in: Ann Oncol. 2018 Oct;29 Suppl 4:iv264-iv266. PMID: 28881921. PubMed
- Amaria R, Prieto P, Tetzlaff M, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018 Feb;19(2):181–193. doi: 10.1016/S1470-2045(18)30015-9. Epub 2018 Jan 18. PMID: 29361468. PubMed
- Eggermont A, Chiarion-Sileni V, Grob J, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016 Nov 10;375(19):1845–55. doi: 10.1056/NEJMoa1611299. Epub 2016 Oct 7. Erratum in: N Engl J Med. 2018 Nov 29;379(22):2185. PMID: 27717298; PMCID: PMC5648545. PubMed
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015 Jan 1;372(1):30-9. doi: 10.1056/NEJMoa1412690. Epub 2014 Nov 16. PMID: 25399551. Journal
- Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015 Mar;7(2):122-36. doi: 10.1177/1758834014566428. PMID: 25755684; PMCID: PMC4346212. PubMed Central
- Chapman P, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011 Jun 30;364(26):2507–16. doi: 10.1056/NEJMoa1103782. Epub 2011 Jun 5. PMID: 21639808; PMCID: PMC3549296. PubMed
- Hauschild A, Grob J, Demidov L, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012 Jul 28;380(9839):358-65. doi: 10.1016/S0140-6736(12)60868-X. Epub 2012 Jun 25. PMID: 22735384. PubMed
- Flaherty K, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012 Jul 12;367(2):107–14. doi: 10.1056/NEJMoa1203421. Epub 2012 Jun 4. PMID: 22663011. PubMed
- Kienstra M, Padhya T. Head and neck melanoma. Cancer Control. 2005 Oct;12(4):242–7. doi: 10.1177/107327480501200406. PMID: 16258496. Journal
- Varey A, Goumas C, Hong A, et al. Neurotropic melanoma: an analysis of the clinicopathological features, management strategies and survival outcomes for 671 patients treated at a tertiary referral center. Mod Pathol. 2017 Nov;30(11):1538–50. doi: 10.1038/modpathol.2017.76. Epub 2017 Jul 21. PMID: 28731051. PubMed
- Strom T, Caudell J, Han D, et al. Radiotherapy influences local control in patients with desmoplastic melanoma. Cancer. 2014 May 1;120(9):1369-78. doi: 10.1002/cncr.28412. Epub 2013 Oct 18. PMID: 24142775; PMCID: PMC4515972. PubMed
- Oliver DE, Patel KR, Switchenko J, et al. Roles of adjuvant and salvage radiotherapy for desmoplastic melanoma. Melanoma Res. 2016 Feb;26(1):35-41. doi: 10.1097/CMR.0000000000000201. PMID: 26397051; PMCID: PMC4869869. PubMed
- Sharabi A, Nirschl C, Kochel C, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015 Apr;3(4):345–55. doi: 10.1158/2326-6066.CIR-14-0196. Epub 2014 Dec 19. PMID: 25527358; PMCID: PMC4390444. PubMed
- Chandra R, Wilhite T, Balboni T, et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology. 2015 May 28;4(11):e1046028. doi: 10.1080/2162402X.2015.1046028. PMID: 26451318; PMCID: PMC4589983. PubMed
- Hiniker S, Reddy S, Maecker H, et al. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys. 2016 Nov 1;96(3):578-88. doi: 10.1016/j.ijrobp.2016.07.005. Epub 2016 Jul 15. PMID: 27681753; PMCID: PMC5077166. PubMed
- Crittenden M, Kohrt H, Levy R, et al. Current clinical trials testing combinations of immunotherapy and radiation. Semin Radiat Oncol. 2015 Jan;25(1):54–64. doi: 10.1016/j.semradonc.2014.07.003. PMID: 25481267; PMCID: PMC4640687. PubMed
- Lee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol. 2011 Apr;164(4):776–84. doi: 10.1111/j.1365-2133.2010.10185.x. Epub 2011 Mar 21. PMID: 21166657. PubMed
- Weber J, Hodi F, Wolchok J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017 Mar;35(7):785–92. doi: 10.1200/JCO.2015.66.1389. Epub 2016 Nov 14. PMID: 28068177. PubMed
- Bray FN, Simmons BJ, Wolfson AH, Nouri K. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther (Heidelb). 2016 Jun;6(2):185–206. doi: 10.1007/s13555-016-0120-y. Epub 2016 Jun 1. PMID: 27250839; PMCID: PMC4906114. PubMed Central
- Ryan J. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012 Mar;132(3 Pt 2):985–93. doi: 10.1038/jid.2011.411. Epub 2012 Jan 5. PMID: 22217743; PMCID: PMC3779131. PubMed
On DermNet
- Melanoma
- Desmoplastic melanoma
- Metastatic melanoma
- Immunotherapy for melanoma
- Ipilimumab
- Nivolumab
- Key clinical-trial evidence for nivolumab
- Targeted cancer therapies
- Trametinib
- Key clinical-trial evidence for trametinib
- Dabrafenib
- Key clinical-trial evidence for dabrafenib
- Vemurafenib
Other websites
- Recent advances in adjuvant therapy for patients with melanoma — European Society for Medical Oncology
- Immunotherapy — Cancer Council
- Medsafe
