Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Nivolumab — extra information
Treatments Lesions (cancerous)
Nivolumab
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet Editor in Chief: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, January 2015. Updated August 2018. Copy edited by Gus Mitchell.
Introduction
Uses
How it works
Administration
Potential drug interactions
Adverse effects
Cutaneous adverse effects
Reporting
Use in pregnancy
Use of nivolumab
Paediatric use
Geriatric use
Renal impairment
Hepatic impairment
What is nivolumab?
Nivolumab (OPDIVO®, Bristol Myers Squibb, New Jersey, USA) is a human programmed death receptor-1 (PD-1)-blocking antibody, approved for treatment of advanced melanoma.
What is nivolumab used for?
In December 2014, the US Food and Drug Administration (FDA) approved the use of nivolumab in:
- Patients with unresectable or metastatic melanoma and disease progression after treatment with ipilimumab (Yervoy®)
- BRAF V600 mutation positive melanoma patients with disease progression despite treatment with ipilimumab and a BRAF inhibitor (eg vemurafenib or dabrafenib).
In October 2015, nivolumab was approved for use in combination with ipilimumab for improved response in advanced melanoma (unresectable or metastatic disease). In January 2016, it was also approved in untreated BRAF-wild type melanoma. In December 2017, the FDA approved nivolumab for the adjuvant treatment of patients with melanoma and involvement of lymph nodes or metastatic disease who have undergone complete resection.
In the European Union, the European Medicines Agency (EMA) has validated for review the Marketing Authorization Application for Nivolumab in advanced melanoma. The application has also been granted accelerated assessment by the EMA’s CHMP (Committee for Medicinal Products for Human Use). Nivolumab is available in New Zealand, and funded by PHARMAC for some cases of metastatic and unresectable advanced melanoma.
In June 2014, the European Commission approved nivolumab a PD-1 immune checkpoint inhibitor, for the treatment of advanced (unresectable or metastatic) melanoma in adults, regardless of BRAF status. The approval allows for the marketing of nivolumab in all 28 Member States of the EU. It follows an accelerated assessment by the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP), which was announced on 24 April, 2015. Approval was based on CheckMate-066 trial demonstrating superior overall survival vs. dacarbazine in the first-line setting and CheckMate-037 trial showing improved response vs. chemotherapy in previously treated patients.
In July 2018, the European Commission (EC) approved nivolumab for the adjuvant treatment of adult patients with melanoma with involvement of lymph nodes or metastatic disease who have undergone complete resection. This indication is for both BRAF mutant and wild-type melanoma patients. With this decision, nivolumab becomes the first and only PD-1 therapy to receive an EC approval in the adjuvant setting. The approval was based on results from the ongoing phase 3 randomized, double-blind CheckMate-238 trial.
Nivolumab is available in New Zealand, and funded by PHARMAC for some cases of metastatic and unresectable advanced melanoma.
How does nivolumab work?
- The PD-1 pathway serves as a checkpoint to limit T-cell–mediated immune responses.
- Cancer cells may exploit “regulatory” pathways, such as checkpoint pathways, to hide from the immune system and shield the tumour from immune attack.
- Both PD-1 ligands, PD-L1 (programmed death receptor ligand 1) and PD-L2, engage the PD-1 receptor and induce PD-1 signaling and associated T-cell “exhaustion,” a reversible inhibition of T-cell activation and proliferation.
- PD-L1/PD-1 interaction inhibits immune activation and reduces T-cell cytotoxic activity when bound.
- This negative feedback loop is essential for maintaining normal immune responses and limits T-cell activity to protect normal cells during chronic inflammation.
- Tumour cells may circumvent T-cell–mediated cytotoxicity by expressing PD-L1 on the tumour itself or on tumour-infiltrating immune cells, resulting in the inhibition of immune-mediated killing of tumour cells.
- Abnormal PD-L1 expression on the surface of melanoma cells activates PD-1 and suppresses cytotoxic T-cell activity.
- This T-cell tolerance enables the tumour cells to avoid recognition and attack by the immune system.
- Nivolumab is a highly selective humanised monoclonal IgG4 antibody that binds to the checkpoint receptor PD-1 on activated T cells. and blocks its interaction with PD-L1 and PD-L2, releasing the PD-1 pathway-mediated inhibition of immune response against tumour cells.
How is nivolumab administered?
- Nivolumab is administered intravenously at a dose of 3 mg/kg every 2 weeks and infused over 1 hr.
- Treatment is continued until disease progression or unacceptable toxicity.
- For nivolumab in combination with ipilumab, nivolumab should be administered first followed by ipilimumab infusion.
- The recommended dose of nivolumab in the combination phase is 1mg/kg administered intravenously over 60 minutes every 3 weeks for the first 4 doses in combination with ipilumab 3mg/kg administered intravenously over 90 minutes. This should be followed by nivolumab monotherapy.
- Nivolumab and ipilumab should be administered and monitored under the supervision of physicians experienced with the use of immunotherapy.
- When OPDIVO is administered in combination with YERVOY (ipilimumab), if either agent is withheld, the other agent should also be withheld.
Dosage modifications
- Hypothyroidism or hyperthyroidism: no recommended dose modifications.
- Renal impairment: no dosage modifications are required.
- Mild hepatic impairment: no dosage modifications required.
- Moderate or severe hepatic impairment: no studies are available.
Withhold medication for any of the following conditions
- Grade 2 pneumonitis
- Grade 2 or 3 colitis
- AST or ALT > 3-5 x upper normal limit (UNL) or total bilirubin > 1.5-3 x UNL
- Serum creatinine > 1.5-6 x UNL or > 1.5 times baseline
- Any other severe or grade 3 treatment-related adverse reactions
- Treatment may be resumed in patients whose adverse reactions recover to grade 0–1.
Permanently discontinue in any of the following conditions
- Any life-threatening or grade 4 adverse reactions
- Grade 3 or 4 pneumonitis
- Grade 4 colitis
- AST or ALT > 5 x UNL or total bilirubin > 3 x UNL
- Serum creatinine > 6 x UNL
- Any severe or grade 3 treatment-related adverse reaction that recurs
- Inability to reduce corticosteroid dose to ≤ 10 mg/day of prednisone or equivalent within 12 weeks
- Persistent grade 2 or 3 treatment-related adverse reactions that do not recover to grade 0–1 within 12 weeks after the last dose.
Link to key clinical-trial evidence about nivolumab
Potential drug interactions with nivolumab
- No formal pharmacokinetic drug-drug interaction studies have been conducted with nivolumab.
- Nivolumab is a human monoclonal antibody and as such is not metabolized by cytochrome P450 (CYP) enzymes or other drug metabolizing enzymes. Inhibition or induction of these enzymes by co-administered medicinal products is not anticipated to affect the pharmacokinetics of nivolumab.
What are the adverse effects of nivolumab?
In clinical trials the following adverse events have been observed at an incidence > 10%: increased alanine aminotransferase (28%), hyponatraemia (25%), increased alkaline phosphatase (22%), rash (21%), pruritus (19%), cough (17%), increased ALT (16%), hyperkalaemia (15%), upper respiratory tract infection (11%).
Other clinically important adverse events occurring at an incidence of 1–10% have included:
- Cardiac disorders: ventricular arrhythmia
- Eye disorders: iridocyclitis
- Infusion-related reactions
- Immune-mediated disorders: severe pneumonitis or interstitial lung disease, colitis, hepatitis, nephritis and thyroid disorders
- In clinical trials, immune-related adverse reactions have occurred at higher frequencies when nivolumab was administered in combination with ipilimumab compared with nivolumab monotherapy.
- Most immune-related adverse reactions improved or resolved with appropriate management, including initiation of corticosteroids and dose modifications.
A clinical trial of the combination of nivolumab and ipilimumab had 36% dropout due to side effects, mainly diarrhoea and elevated liver function tests.
Cutaneous adverse effects of nivolumab
Skin complications are common in patients treated with nivolumab, with about 40% of patients experiencing these, with mean onset of rash being about 10 months into the course of treatment. Reported reactions include:
- Morbilliform eruption
- Pruritus
- Lichenoid eruption
- Psoriasiform reactions
- Eczema
- Drug-induced vitiligo and poliosis
- Stomatitis
- Bullous pemphigoid
- Sarcoid-like granuloma
- Multiple keratoacanthomas.
Darkening of hair has been reported in a few patients taking PD-1 inhibitors for non-small cell lung cancer [1].
See also Cutaneous adverse effects of checkpoint inhibitors.
Reporting of suspected adverse reactions
For continued monitoring of the benefit and risk balance of nivolumab, healthcare professionals are asked to report any suspected adverse reactions to the New Zealand Pharmacovigilance Centre.
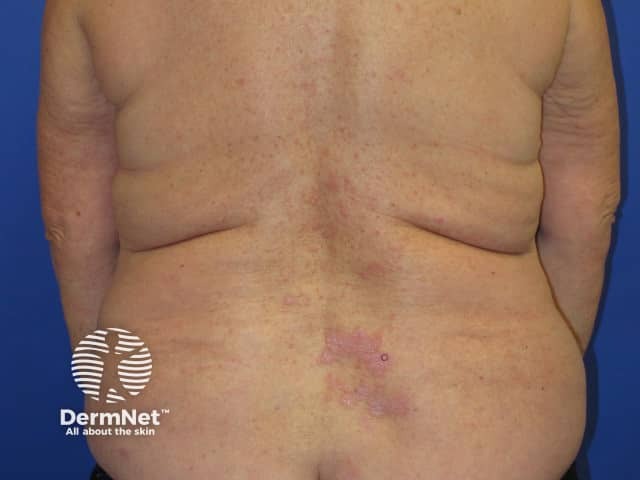
Nivolumab-induced lichenoid dermatitis
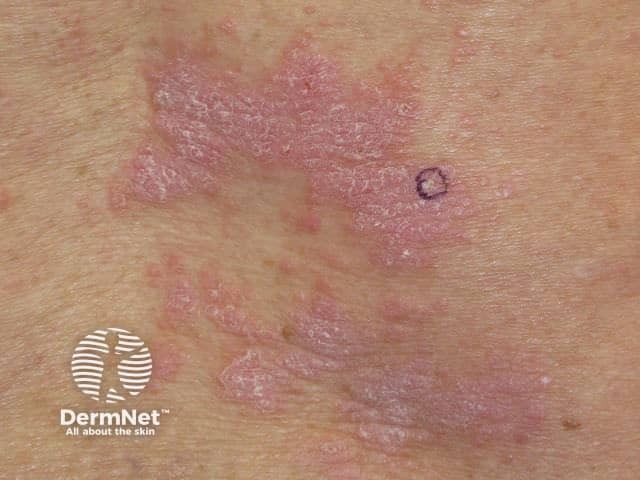
Nivolumab-induced lichenoid dermatitis
Use of nivolumab in pregnancy
- The incidences of malformations and pregnancy loss in human pregnancies have not been established for nivolumab.
- Nivolumab should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- In animal reproduction studies, administration to cynomolgus monkeys from the onset of organogenesis through delivery resulted in increased abortion and premature fetal death.
- Human IgG4 is known to cross the placental barrier and nivolumab is an IgG4; therefore, nivolumab has the potential to be transmitted from the mother to the developing fetus.
- The effects of nivolumab are likely to be greater during the second and third trimesters of pregnancy; there are no available human data informing the drug-associated risk.
Use of nivolumab in nursing mothers
- It is not known whether nivolumab or its metabolites are present in human milk.
- Advise women to discontinue breastfeeding during treatment.
Paediatric use of nivolumab
The safety and effectiveness of nivolumab has not been established in paediatric patients. Nivolumab should not be used in children below 18 years of age.
Geriatric use of nivolumab
Clinical studies of nivolumab did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently from younger patients. No overall differences in safety or efficacy were reported between elderly (≥ 65 years) and younger patients (< 65 years). No dose adjustment is required for elderly patients (≥ 65 years).
Renal impairment and nivolumab
Based on a population pharmacokinetic analysis, no dose adjustment is recommended in patients with renal impairment.
Hepatic impairment and nivolumab
Based on a population pharmacokinetic analysis, no dose adjustment is recommended for patients with mild hepatic impairment. Nivolumab has not been studied in patients with moderate or severe hepatic impairment.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Rivera N, Boada A, Bielsa MI, Fernández-Figueras MT, Carcereny E, Moran MT, Ferrándiz C. Hair Repigmentation During Immunotherapy Treatment With an Anti–Programmed Cell Death 1 and Anti–Programmed Cell Death Ligand 1 Agent for Lung Cancer. JAMA Dermatol. Published online July 12, 2017. doi:10.1001/jamadermatol.2017.2106. Journal
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–33. Journal
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumour remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32: 1020–30. PubMed
- Robert C, Long GV, Brady B, et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N Engl J Med 2015; 372: 320–30. Journal
- Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16: 375–84. PubMed
- Feldstein SI, Patel F, Larsen L, Kim E, Hwang S, Fung MA. Eruptive keratoacanthomas arising in the setting of lichenoid toxicity after programmed cell death 1 inhibition with nivolumab. J Eur Acad Dermatol Venereol 2017; 32: e58–e59. doi: 10.1111/jdv.14503. PubMed
On DermNet
- Key clinical-trial evidence about nivolumab
- Melanoma
- BRAF inhibitors: vemurafenib and dabrafenib
- Ipilumimab
- Genetics of melanoma
- Drug-induced vitiligo
- Targeted therapy
- Cutaneous adverse effects of anti-melanoma therapies
- Cutaneous adverse effects of checkpoint inhibitors
- Adverse cutaneous reactions to drugs
Other websites
- Prescribing information (Nivolumab, US Label)
- Nivolumab — European Medicines Agency CHMP Positive Opinion
- Nivolumab for treating advanced (unresectable or metastatic) melanoma — NICE technology appraisal guidance, February 2016
- Nivolumab in combination with ipilimumab for treating advanced melanoma — NICE technology appraisal guidance, July 2016
- Nivolumab — Medsafe Data Sheet
- New Zealand Pharmacovigilance Centre
- Immunotherapy What to expect videos
