Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Cutaneous adverse effects of checkpoint inhibitors — extra information
Cutaneous adverse effects of checkpoint inhibitors
Author: Dr Harmony Thompson, Medical Registrar, Hamilton, New Zealand. DermNet Editor in Chief: Adjunct A/Prof. Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. June 2020.
Introduction
Immune adverse-events
Definition - cutaneous adverse effects
Differential diagnoses
Diagnosis
Treatment
Outcome
What is a checkpoint inhibitor?
A checkpoint inhibitor is a form of immunotherapy used to treat cancer that targets the immune response. It enhances the immune system by inhibiting the ‘brake’ on an activated immune system (cytotoxic T-lymphocyte-associated antigen 4 [CTLA-4] inhibitors such as ipilimumab, or by blocking the immune inhibitory expression by the cancer cells (programmed cell death protein 1 [PD1] such as pembrolizumab and nivolumab, or PD-L1 inhibitors such as avelumab). Immune checkpoints are essential in maintaining immune homeostasis by augmenting or inhibiting immune responses and allowing tolerance to self-antigens [1–4].
Checkpoint inhibitors can cause a wide spectrum of side effects due to their triggering cytotoxic CD4+/CD8+ T-cell activation. More than 60% of patients develop immune-related adverse events, which can theoretically affect any organ [1–4].
What are the immune-related adverse events?
Immune-related adverse events associated with checkpoint inhibitors include:
- Autoimmune skin diseases and mucosal toxicity (eg, stomatitis)
- Fatigue
- Infusion-related reactions
- Diarrhoea and colitis
- Hepatitis
- Pneumonitis
- Endocrinopathies
- Thyroiditis
- Hypophysitis
- Adrenal insufficiency
- Type 1 diabetes.
Other reactions include:
- Effects on the kidney
- Acute kidney injury
- Nephritis
- Effects on the exocrine pancreas
- Pancreatitis
- Neurological symptoms
- Polyneuritis
- Aseptic meningitis
- Myasthenia gravis
- Posterior reversible encephalopathy syndrome
- Cardiovascular disease
- Cardiotoxicity
- Thromboembolism
- Haematological abnormalities
- Red cell aplasia
- Neutropenia
- Thrombocytopenia
- Acquired haemophilia
- Cryoglobulinaemia
- Ocular symptoms
- Uveitis
- Episcleritis
- Conjunctivitis
- Rheumatological disease
- Arthritis
- Sicca syndrome
- Vasculitis
- Musculoskeletal disease
- Myositis [1,4].
What are the cutaneous adverse effects of checkpoint inhibitors?
Adverse effects involving the skin are among the most common immune-related adverse events due to checkpoint inhibitors, occurring in 30–60% of patients. Various presentations are described below [5–8].
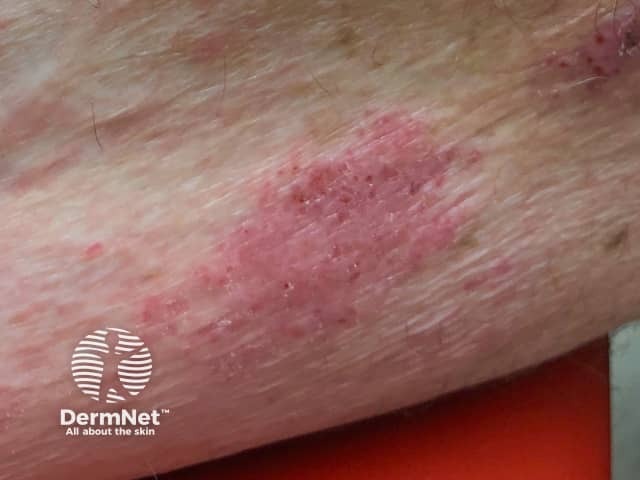
Dermatitis induced by pembrolizumab
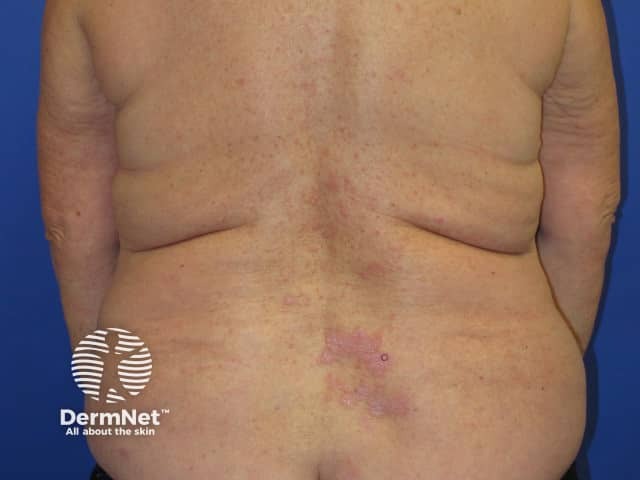
Nivolumab-induced lichenoid dermatitis
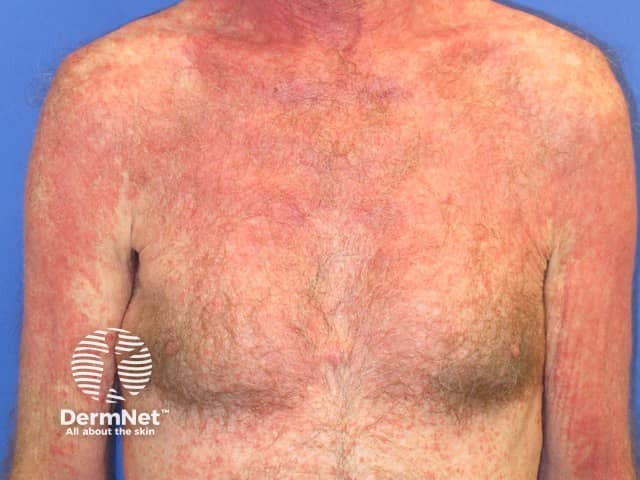
Pembrolizumab-induced psoriasis
Dermatitis
Dermatitis often starts after the first few cycles of treatment, with worsening after each subsequent cycle of treatment. Delayed eruptions can also occur.
- The incidence is 14–24%.
- Itchy rash occurs on trunk and limbs with sparing of the face.
Lichenoid drug reaction
Lichenoid drug reactions start after several weeks to months of treatment.
- Itchy plaques mainly occur on trunk and limbs.
Psoriasis
Psoriasis develops several months after treatment.
- It mainly affects patients who have previously been diagnosed with psoriasis but de novo psoriasis also occurs.
- It can present as:
See more on immunotherapy-related psoriasiform reactions.
SCARs
Severe and life-threatening cutaneous reactions (SCARs) include:
- Extensive exfoliative dermatitis
- Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN)
- Acute generalised exanthematous pustulosis (AGEP)
- Drug hypersensitivity syndrome
- Bullous pemphigoid and other autoimmune blistering skin diseases.
Vitiligo
Vitiligo can present as localised or widespread areas of depigmentation.
- Vitiligo tends to present as symmetrical and bilateral white patches.
- Patches of vitiligo may surround scars or cutaneous metastases.
- It can persist after discontinuation of treatment.
Eosinophilic fasciitis
Pembrolizumab and nivolumab are being reported to trigger eosinophilic fasciitis.
Other skin diseases associated with checkpoint inhibitors include:
- Acneiform eruptions
- Acantholytic dermatosis
- Urticaria
- Xerosis
- Photosensitivity
- Dermatomyositis
- Alopecia areata
- Sarcoidosis
- Nail dystrophy
- Stomatitis
- Dermatitis herpetiformis
- Cutaneous vasculitis
- Acute neutrophilic dermatosis.
What is the differential diagnosis for cutaneous adverse effects of checkpoint inhibitors?
It can be difficult to distinguish between the adverse effects of immunotherapy and primary skin disease. It is important to rule out infection and the adverse cutaneous effects of other drugs [3].
How are cutaneous adverse effects on checkpoint inhibitors diagnosed?
The specific diagnosis may be clear by taking a history and performing a careful examination.
Possible investigations for patients with skin problems on checkpoint inhibitors include:
- Full blood count
- Liver function
- Kidney function
- Tryptase
- Immunoglobulin (Ig) E levels
- C-reactive protein (CRP) if suspected infection
- Antinuclear antibody test, anti-Ro, anti-La, double-stranded DNA, and antihistone, if suspecting cutaneous lupus erythematosus or dermatomyositis.
A skin biopsy may be helpful to differentiate various inflammatory rashes.
What is the treatment for dermatitis secondary to checkpoint inhibitors?
To decide on treatment, the clinical severity of a skin problem needs to be carefully assessed in conjunction with the impact on activities of daily living. Dermatologist input and skin biopsy should be obtained prior to commencing systemic corticosteroids (which are contraindicated for psoriasis and ineffective for vitiligo).
There are four grades of severity.
Grade 1
Symptoms in Grade 1 include:
- An inflammatory rash with no effect on the quality of life
- An asymptomatic bullous dermatosis affecting < 10% body surface area (BSA).
To alleviate these symptoms, continue immunotherapy, treat the rash with moisturisers and sun protection, and avoid skin irritants such as soap. Consider the use of mild to moderate-potency topical steroids.
Grade 2
Symptoms in Grade 2 include:
- An inflammatory rash that affects the quality of life
- A bullous dermatosis affecting 10–30% BSA and affecting the quality of life
- A SCAR affecting 10–30% BSA without mucosal involvement.
To alleviate these symptoms, consider interrupting immunotherapy until the rash has reverted to grade 1. Treat the rash with moisturisers and sun protection, and avoid skin irritants such as soap. Consider high to very high-potency topical steroids, or systemic corticosteroids (eg, prednisone 0.5–1 mg/kg/day).
Grade 3
Symptoms in Grade 3 include:
- An inflammatory rash that has failed to respond to prior interventions
- A bullous dermatosis affecting > 30% BSA, limiting the self-care activities of daily living
- A SCAR affecting < 10% BSA with mucosal involvement.
Consider withholding immunotherapy and consult with dermatologist. Treat the rash with moisturisers and sun protection, and avoid skin irritants such as soap. Consider high to very high-potency topical steroids, and systemic corticosteroids (eg, prednisone 0.5–1 mg/kg/day or IV methylprednisolone 1–2 mg/kg/day with slow tapering).
Grade 4
Symptoms in Grade 4 include:
- An intolerable or life-threatening rash that is unmanageable with prior interventions
- A bullous dermatosis affecting > 30% BSA with fluid or electrolyte abnormalities
- A SCAR affecting > 10–30% BSA with mucous membrane involvement or associated with systemic symptoms or blood test abnormalities.
It is recommended to immediately stop and permanently discontinue immunotherapy if Grade 4 symptoms are present, and a dermatologist should be consulted. Treat the rash with moisturisers and sun protection, and avoid skin irritants such as soap. Consider high to very high-potency topical steroids. Consider IV methylprednisolone 1–2 mg/kg/day with slow tapering.
If immunotherapy should be suspended, then only resume when the steroid dose is < 10 mg/day of prednisone equivalent [1–8].
Other possible treatments
Other possible treatments for any dermatosis secondary to checkpoint inhibitors can include:
- Oral antihistamines for pruritus (these are most effective for urticaria)
- Phototherapy for lichenoid reactions and psoriasis
- Topical vitamin D analogues (calcipotriol) for psoriasis
- Methotrexate for dermatitis, lichenoid reactions, and psoriasis
- Hydroxychloroquine for sarcoidosis or lichenoid reactions
- Antibiotics for suspected secondary bacterial infection
- Intravenous immunoglobulin or ciclosporin for SJS/TEN
- Rituximab and omalizumab for bullous pemphigoid or other blistering diseases.
A multidisciplinary approach is necessary, with close communication between oncologists, dermatologists, and the patient. The treatment of immune-related immune reactions does not appear to affect the cancer response to checkpoint inhibitors. Note that prolonged immunosuppressive treatment increases the risk of opportunistic infections.
What is the outcome for a skin disease caused by a checkpoint inhibitor?
Most cutaneous adverse reactions to checkpoint inhibitors are mild (Grade 1) and resolve with topical treatment. SCARs are rare but can be life-threatening and may require admission to an intensive care unit.
Rashes due to immunotherapy can often take a long time to resolve and can persist for months after immunotherapy is discontinued.
References
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies [published correction appears in Ann Oncol. 2016 Jul;27(7):1362]. Ann Oncol. 2015;26(12):2375-91. doi:10.1093/annonc/mdv383. PubMed
- Kähler KC, Hassel JC, Heinzerling L, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14(7):662-81. doi:10.1111/ddg.13047. PubMed
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-68. doi:10.1200/JCO.2017.77.6385. PubMed
- Hryniewicki AT, Wang C, Shatsky RA, Coyne CJ. Management of immune checkpoint inhibitor toxicities: a review and clinical guideline for emergency physicians. J Emerg Med. 2018;55(4):489-502. doi:10.1016/j.jemermed.2018.07.005. PubMed
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors : skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345-61. doi:10.1007/s40257-017-0336-3. PubMed
- To S, Lee C, Chen Y, et al. Psoriasis Risk With Immune Checkpoint Inhibitors. JAMA Dermatol. 2025;161(1):31–38. PubMed
- de Golian E, Kwong BY, Swetter SM, Pugliese SB. Cutaneous complications of targeted melanoma therapy [published correction appears in Curr Treat Options Oncol. 2016 Dec;17 (12 ):63]. Curr Treat Options Oncol. 2016;17(11):57. doi:10.1007/s11864-016-0434-0. PubMed
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010. PubMed
- Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221. PubMed
- Chan KK, Magro C, Shoushtari A, et al. Eosinophilic fasciitis following checkpoint inhibitor therapy: four cases and a review of literature. Oncologist. 2020;25(2):140-9. doi:10.1634/theoncologist.2019-0508. PubMed
On DermNet
- Targeted cancer therapies
- Ipilimumab
- Pembrolizumab
- Nivolumab
- Cutaneous adverse effects of anti-melanoma therapies
- Immunotherapy-related psoriasiform reactions
- Adverse cutaneous reactions to drugs
Other websites
- Pembrolizumab — New Zealand Medsafe datasheet
- Pembrolizumab for treating advanced melanoma after disease progression with ipilimumab — UK National Institute for Health and Care Excellence technology appraisal guidance. Updated September 2017
- KEYTRUDA® (pembrolizumab) for injection, for intravenous use — US FDA prescribing information, September 2014 (pdf)
- Ipilimumab for previously treated advanced (unresectable or metastatic) melanoma — NICE technology appraisal guidance, Dec 2012
- Ipilimumab for previously untreated advanced (unresectable or metastatic) melanoma — NICE technology appraisal guidance, July 2014
- Immune Checkpoint Inhibitors and Their Side Effects — American Cancer Society
- Checking in on the adverse reactions to checkpoint inhibitors — American Academy of Dermatology
- Stage 4 Melanoma — British Academy of Dermatology
