Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Key clinical-trial evidence for pembrolizumab — extra information
Key clinical-trial evidence for pembrolizumab
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet Editor in Chief: Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. First published December 2014; updated March 2018. Copy edited by Gus Mitchell/Maria McGivern.
This article was updated with financial support from Merck Sharp & Dohme (New Zealand) Limited, distributors of Keytruda® in New Zealand. Sponsorship does not influence content.
Introduction
The KEYNOTE-001 study
The KEYNOTE-002 study
The KEYNOTE-006 study
Conclusions
Introduction
Pembrolizumab (Keytruda®) is a highly selective humanised monoclonal antibody directed against the programmed cell death 1 (PD-1) receptor on T cells. The drug blocks the PD-1 receptor, thereby preventing the formation of programmed cell death ligand 1 (PD-L1) complexes. This mechanism causes the activation of T-cell mediated immune responses against tumour cells.
On 4 September 2014, the US Food and Drug Administration (FDA) approved pembrolizumab as a breakthrough therapy for the treatment of metastatic melanoma, based on response rates demonstrated in clinical trial data from 173 patients with melanoma in a cohort of the KEYNOTE-001 study.
Since then results from two further clinical trials (KEYNOTE-002 and KEYNOTE-006) have continued to show the benefit of pembrolizumab for the treatment of advanced unresectable or metastatic melanoma.
Pembrolizumab is now registered for the treatment of advanced (metastatic or unresectable) melanoma in many countries worldwide, including Europe, the UK, and New Zealand.
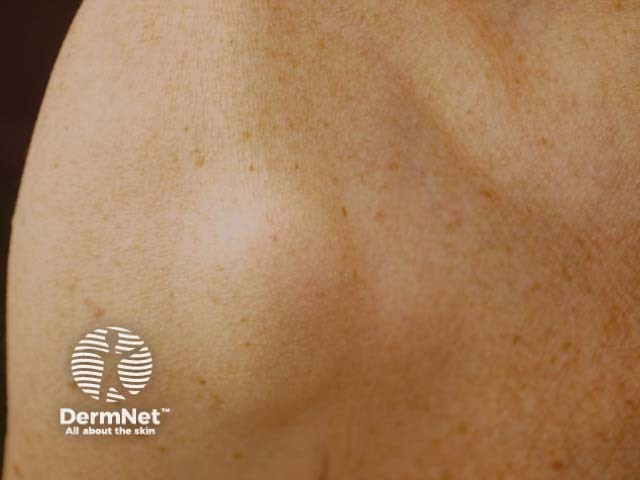
Subcutaneous metastatic melanoma
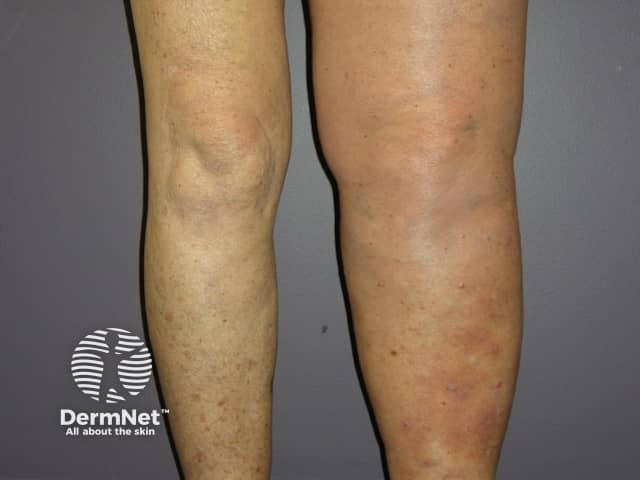
Metastatic melanoma
The KEYNOTE-001 study
- The efficacy of pembrolizumab was investigated in a multicentre, open-label, randomised (1:1), dose-comparative Phase 1b study.
- The key eligibility criteria were unresectable or metastatic melanoma with progression of disease and refractory to treatment with two or more doses of ipilimumab (3 mg/kg or higher), and, if BRAF V600 mutation-positive, a BRAF or MEK inhibitor, and disease progression within 24 weeks following the last dose of ipilimumab.
- Patients were randomised to receive 2 mg/kg n = 89 or 10 mg/kg n = 84 of pembrolizumab every 3 weeks until unacceptable toxicity or disease progression that was symptomatic.
- Assessment of tumour status was performed 12 weeks after the first dose of pembrolizumab and every 12 weeks thereafter.
- The major efficacy outcome measures were duration of response and confirmed overall response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1) as assessed by a blinded independent central review.
- The ORR was 24% (95% CI: 15–34) in the 2 mg/kg arm, consisting of one complete response and 20 partial responses.
- Among the 21 patients with an objective response, three (14%) had disease progression at 2.8, 2.9, and 8.2 months, respectively, after the initial response.
- The duration of response in the remaining 18 patients (86%) varied from 1.4 to 8.5 months and included eight patients with ongoing responses of 6 months or longer.
- Similar ORR results were observed in the 10 mg/kg arm.
- The response rate did not differ significantly between patients who had received prior ipilimumab treatment and those who had not.
- Table 1 presents adverse reactions identified from analyses of the 89 patients with unresectable or metastatic melanoma who received pembrolizumab 2 mg/kg every 3 weeks.
- The median duration of exposure to pembrolizumab was 6.2 months (range: 1 day to 15.3 months) with a median of nine doses (range: 1–23).
- Pembrolizumab was discontinued in 6% of the 89 patients because of adverse reactions.
Table 1. Drug-related adverse reactions occurred in > 10% of patients in KEYNOTE-001
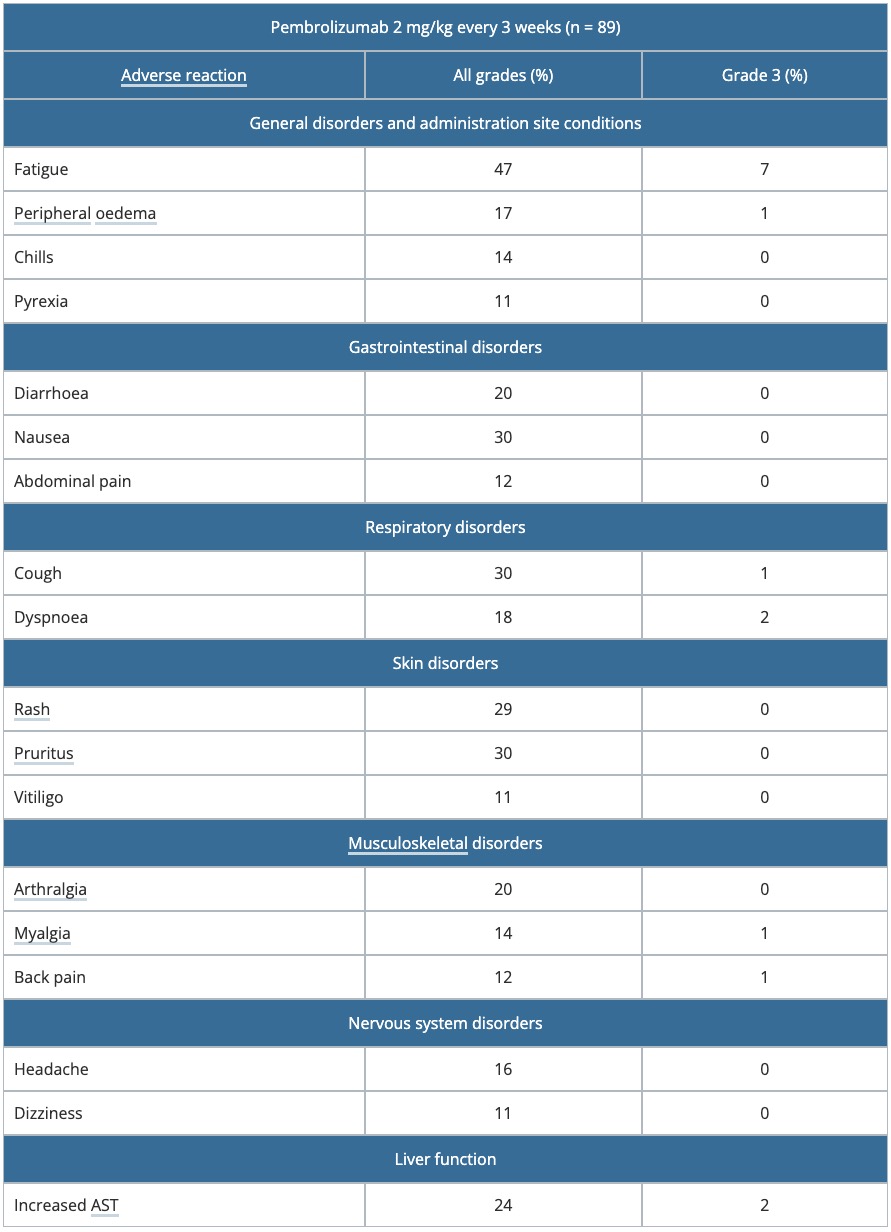
AST: aspartate transaminase
The KEYNOTE-002 study
Efficacy
- KEYNOTE-002 was a Phase II study that assessed the efficacy and safety of two pembrolizumab doses compared with investigator-choice chemotherapy in patients with ipilimumab-refractory melanoma.
- Patients had histologically or cytologically confirmed unresectable stage III or stage IV melanoma that had progressed within 24 weeks of the last ipilimumab dose (minimum two doses, 3 mg/kg once every 3 weeks) and/or had had previous BRAF or MEK inhibitor therapy, or both (if mutant-positive).
- Patients were randomised (1:1:1) to receive intravenous pembrolizumab 2 mg/kg or 10 mg/kg every 3 weeks or investigator-choice chemotherapy (paclitaxel plus carboplatin, paclitaxel, carboplatin, dacarbazine, or oral temozolomide).
- Tumour assessments were done before starting study treatment (baseline), at Week 12, every 6 weeks through to Week 48, and then every 12 weeks thereafter.
- The primary endpoint was progression-free survival, the time from randomisation to first documented disease progression per RECIST v1.1 by an independent central review, or death from any cause, whichever occurred first.
- Results are summarised in Table 2.
Table 2. Summary of efficacy parameters in KEYNOTE-002.
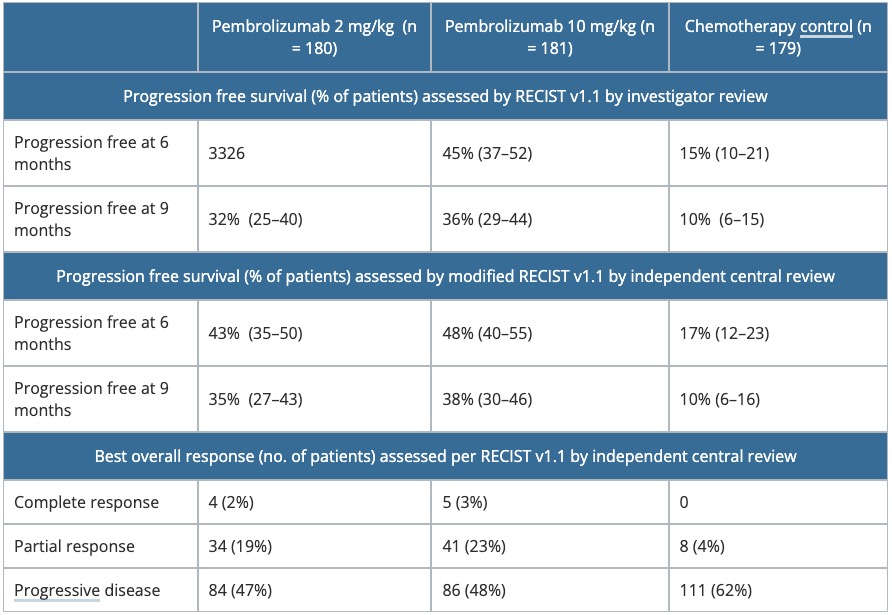
RECIST, Response Evaluation Criteria in Solid Tumors
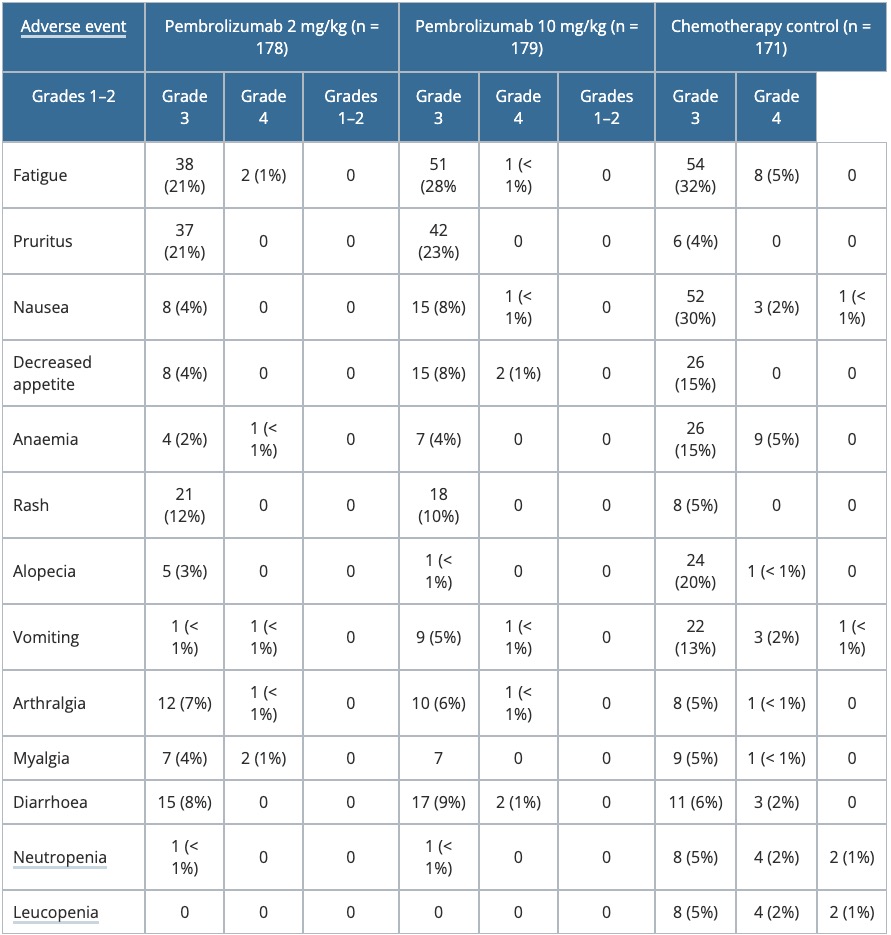
Adverse events
- The incidence of Grades 3–4 drug-related adverse events in KEYNOTE-002 higher in patients treated with chemotherapy compared to those treated with pembrolizumab.
- The most common treatment-related adverse events with chemotherapy were alopecia, anaemia, decreased appetite, fatigue, nausea, and vomiting.
- The treatment-related adverse events that were more frequent with pembrolizumab were rash, pruritus, and diarrhoea.
- Table 3 is a summary of adverse events in KEYNOTE-002.
Table 3. Summary of adverse events — KEYNOTE-002
Health-related quality of life
- Health-related quality of life (HRQoL) was assessed using the Global Health Status (GHS)/HRQoL scores of the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire-Core 30 (EORTC QLQ-C30) at baseline, Weeks 3, 6, 12, 24, and 36, at treatment discontinuation, and during safety follow-up.
- HRQoL was maintained to a greater degree with pembrolizumab versus chemotherapy, with fewer patients treated with pembrolizumab experiencing deterioration in GHS at Week 12 (31.8% patients with pembrolizumab 2 mg/kg, 26.6% for 10 mg/kg, and 38.3% for chemotherapy).
- Results are summarised in Table 4.
- In addition to the GHS/HRQoL score, patients in the two pembrolizumab arms had consistently smaller longitudinal score changes from baseline to Week 12 across functional scales, including physical, cognitive, social, and emotional functions, and across symptoms scales including fatigue, nausea/vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, and diarrhoea.
Table 4. Change from baseline to Week 12 in the GHS score of the EORTC QLQ-C30 in KEYNOTE-002
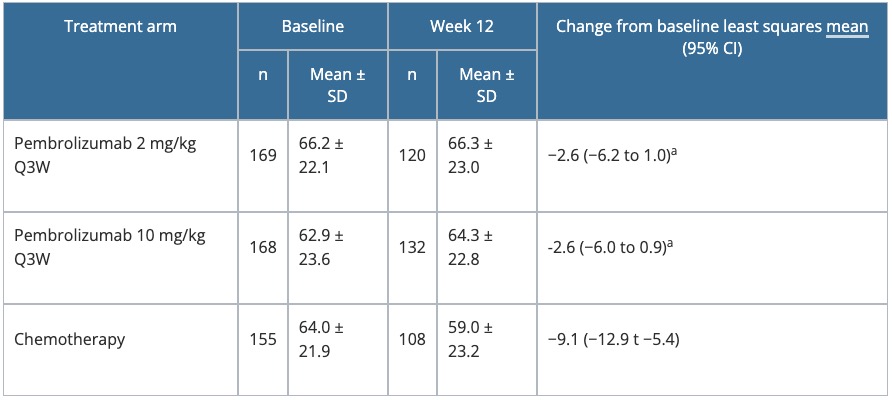
a P = 0.01 versus chemotherapy.
The KEYNOTE-006 study
Efficacy
- The Phase III KEYNOTE-006 study showed superior overall and progression-free survival of pembrolizumab versus ipilimumab in patients with advanced melanoma.
- 834 patients with advanced melanoma were enrolled and randomly assigned to receive intravenous pembrolizumab every 2 weeks (n = 279), intravenous or every 3 weeks (n = 277), or intravenous ipilimumab every 3 weeks (n = 278).
- Median follow-up was 22.9 months.
- The estimated 6-month progression-free survival rates were 47.3% for patients receiving pembrolizumab every 2 weeks, 46.4% for those receiving pembrolizumab every 3 weeks, and 26.5% for those receiving ipilimumab.
- One-year estimates of overall survival were 74.1% for patients receiving pembrolizumab every 2 weeks (hazard ratio for death as compared with the ipilimumab group, 0.63; 95% CI: 0.47–0.83; p < 0.0005), 68.4% for those receiving pembrolizumab every 3 weeks (hazard ratio for death as compared with the ipilimumab group, 0.69; 95% CI: 0.52–0.90; p = 0.0036), and 58.2% for those receiving ipilimumab.
- 24-month overall survival rate was 55% in the 2-week group, 55% in the 3-week group, and 43% in the ipilimumab group.
Health-related quality of life
- The primary patient-related outcome assessment in KEYNOTE-006 was the score change from baseline to Week 12 in EORTC QLQ-C30 GHS/HRQoL score between treatment arms, using a constrained longitudinal data analysis.
- From baseline to Week 12, GHS/HRQoL scores were better maintained with pembrolizumab than with ipilimumab (decrease of −1.9 and −2.5 for pembrolizumab vs −10.0 for ipilimumab; p < 0.001 for each pembrolizumab arm vs ipilimumab; see Table 5).
- Fewer patients treated with pembrolizumab experienced deterioration in GHS at Week 12 (31% for pembrolizumab every 2 weeks; 29% for pembrolizumab every 3 weeks, and 44% for ipilimumab), with similar trends being observed for individual functioning and symptoms scales.
Table 5. Change from baseline to Week 12 in the GHS score of the EORTC QLQ-C30 — KEYNOTE-006
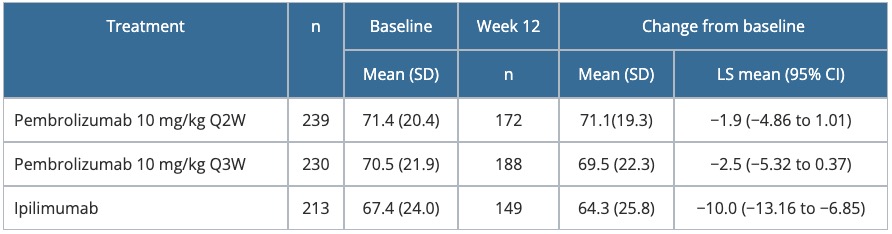
CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire-Core 30; GHS, Global Health Status; LS, least squares; Q2W, every 2 weeks; Q3W, every 3 weeks; SD, standard deviation
Adverse events
- In KEYNOTE-006, the most common treatment-related adverse events of any grade occurring in the pembrolizumab groups were fatigue (20.9% in the 2-week group and 19.1% in the 3-week group), diarrhoea (16.9% and 14.4%, respectively), rash (14.7% and 13.4%, respectively), and pruritus (14.4% and 14.1%, respectively).
- For ipilimumab, the most frequent adverse events were pruritus (25.4%), diarrhoea (22.7%), fatigue (15.2%), and rash (14.5%).
Conclusions
- The findings of the KEYNOTE-001, KEYSTONE-002 and KEYSTONE-006 trials establish pembrolizumab as a new standard of care for melanoma and support the accelerated approval granted by the US FDA for the use of pembrolizumab in patients with unresectable or metastatic melanoma whose disease had progressed after ipilimumab and, if BRAF V600 mutant-positive, a BRAF inhibitor.
- HRQoL was better maintained with pembrolizumab than with chemotherapy (KEYNOTE-002) or ipilimumab (KEYNOTE-006), further supporting the use of pembrolizumab in patients with ipilimumab-refractory melanoma.
- Overall, the combination of prolonged progression-free survival and overall survival, a decreased incidence of high-grade toxicity, and a better HRQoL provided by pembrolizumab compared with ipilimumab or chemotherapy, supports the use of this drug as a standard of care for patients with advanced melanoma.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014 Sep 20; 384(9948):1109-17. DOI: 10.1016/S0140-6736(14)60958-2.PubMed
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369: 134–44. DOI: 10.1056/NEJMoa1305133.PubMed
- Poole RM. Pembrolizumab: first global approval. Drugs 2014; 74: 1973–81. DOI: 10.1007/s40265-014-0314-5. PubMed
- Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer 2017; 86: 37–45. DOI: 10.1016/j.ejca.2017.07.022.PubMed
- Schadendorf D, Dummer R, Hauschild A, et al. Health-related quality of life in the randomised KEYNOTE-002 study of pembrolizumab versus chemotherapy in patients with ipilimumab-refractory melanoma. Eur J Cancer 2016; 67: 46–54. DOI: 10.1016/j.ejca.2016.07.018.PubMed
- Petrella TM, Robert C, Erica Richtig, et al. Patient-reported outcomes in KEYNOTE–006, a randomised study of pembrolizumab versus ipilimumab in patients with advanced melanoma. Eur J Cancer 2017; 86: 115–24. DOI: 10.1016/j.ejca.2017.08.032.PubMed
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–32. PubMed
- Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017; 390(10105): 1853–62. DOI: 10.1016/S0140-6736(17)31601-X.PubMed
- Gangadhar TC, Hwu WJ, Postow MA, et al. Efficacy and safety of pembrolizumab in patients enrolled in KEYNOTE-030 in the United States: an expanded access program. J Immunother 2017; 40: 334–40. DOI: 10.1097/CJI.0000000000000186.PubMed
On DermNet
Other websites
- KEYTRUDA® (pembrolizumab) for injection, for intravenous use — US FDA prescribing information, September 2014 (pdf)
- Keytruda — Medsafe New Zealand manufacturer datasheet (pdf)
- Cancer treatment with Keytruda — Information for patients in New Zealand provided by the manufacturer
- Keytruda® (pembrolizumab) Healthcare Professionals Portal — MSD Oncology
