Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Cutaneous adverse effects of targeted anticancer therapies — extra information
Reactions Treatments Lesions (cancerous)
Cutaneous adverse effects of targeted anticancer therapies
Authors: Sarah Friske and Dr Riyad N.H. Seervai, Baylor College of Medicine, United States of America. Copy edited by Gus Mitchell. July 2022.
Introduction
Introduction - targeted anticancer therapies
Targets
Cutaneous adverse effects
Introduction
The advent of targeted cancer therapies, including monoclonal antibodies and small-molecule inhibitors, has initiated an era of precision medical oncology focused on targeting vulnerabilities specific to cancer cells.
All modalities of anticancer therapy have the risk of causing substantial cutaneous toxicities that can seriously affect a patient’s quality of life.
What are targeted anticancer therapies?
Traditional chemotherapy utilises nonspecific properties of cytotoxic agents to impede cell division. Alternatively, the discovery of cancer-associated gene mutations and hallmark-related signal transduction pathways has allowed physicians to treat cancer in a more precise manner.
What do these drugs target?
Monoclonal antibodies
Monoclonal antibodies (Mabs) target factors related to the induction of apoptosis and cell death, and prevention of angiogenesis and growth factor signalling. Recently, Mabs have been effective at leveraging the immune system to avert cancer through immune checkpoint inhibition.
Small molecule inhibitors
An assortment of small-molecule inhibitors (Nibs) have been approved to target pathways associated with the most distinctive features of cancer leading to superior overall/progression-free survival outcomes and a reduced adverse-effect profile compared with that of chemotherapy.
What are the cutaneous adverse effects of targeted therapies?
MAP Kinase Pathway
The mitogen-activated protein (MAP) kinase pathway promotes cancer survival, proliferation, angiogenesis, migration, and invasion. Frequent anticancer targets of this pathway include the protein kinases BRAF and MEK.
BRAF inhibitors
BRAF mutations are found in ~50% of melanomas. The first two BRAF inhibitors, vemurafenib and dabrafenib, are associated with significant improvement in progression-free and overall survival in patients with metastatic melanoma.
Adverse cutaneous reactions include:
- Actinic keratosis
- Alopecia
- Acneiform,
- Basal cell carcinoma
- Cheilitis
- Cysts
- Eczematous and maculopapular rashes.
- Grover disease
- Hair changes
- Hand-foot reaction
- Keratoacanthoma
- Keratosis pilaris
- Melanocyte changes
- Nail changes
- Panniculitis
- Papillomas
- Photosensitivity
- Pruritus
- Seborrheic keratosis
- Stomatitis/mucositis
- Verrucae
- Vitiligo
- Xerostomia.
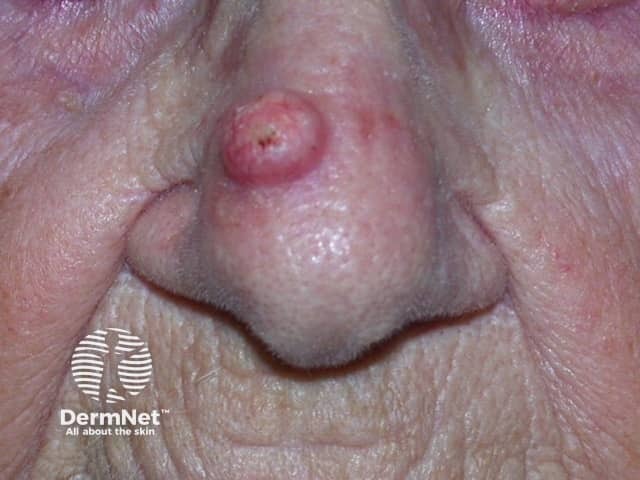
Keratoacanthoma
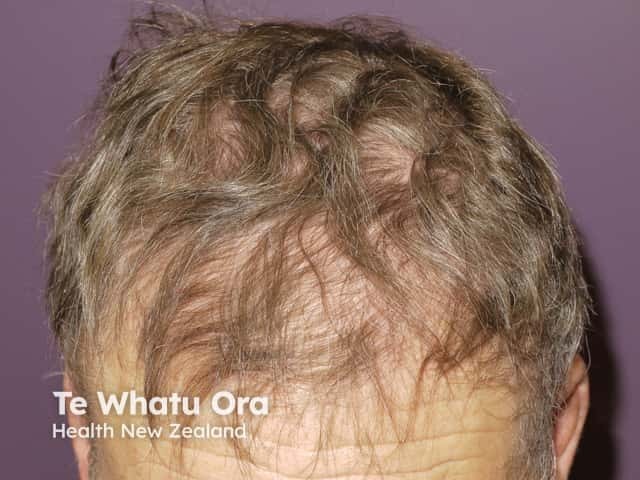
Alopecia due to vemurafenib
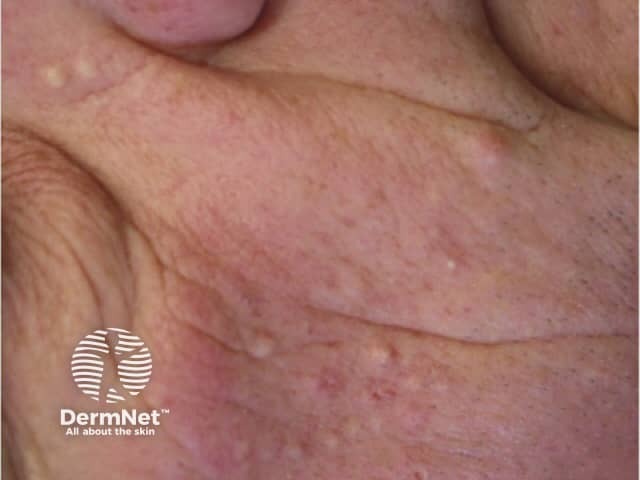
Folliculitis due to vemurafenib
MEK inhibitors
Trametinib, selumetinib, cobimetinib, and binimetinib are MEK inhibitors currently in use.
Adverse cutaneous reactions include:
- Alopecia
- Nail changes
- Pruritus
- Stomatitis/mucositis
- Cheilitis
- Xerostomia
- Acneiform rashes.
Receptor Tyrosine Kinase Signaling
Anticancer therapies target receptor tyrosine kinase signalling pathways via inhibition of multikinases VEGF, EGFR, and FGFR.
Multikinase Inhibitors
Multikinase inhibitors currently include sorafenib, sunitinib, axitinib, regorafenib, lenvatinib, and vandetanib.
Adverse cutaneous reactions include:
- Erythematous eruption/desquamation
- Scalp/facial erythema or dysesthesia
- Hand-foot skin reaction
- Alopecia
- Splinter and subungual haemorrhages
- Xerosis
- Pruritus
- Stomatitis
- Keratosis pilaris
- Keratoacanthomas
- Skin discoloration
- Hair discoloration
- Photosensitivity
- Maculopapular and acneiform skin rashes.
VEGF Inhibitors
VEGF is key in moderating tumorigenesis by promoting the development of abnormal vasculature around the tumour. Current therapies targeting VEGF include bevacizumab, regorafenib, and lenvatinib.
The most common dermatologic toxicities are:
- Hand-foot skin reaction
- Maculopapular rash
- Mucositis
- Alopecia
- Stomatitis
- Dry mouth
- Pruritus
- Xerosis
- Blistering
- Photosensitivity.
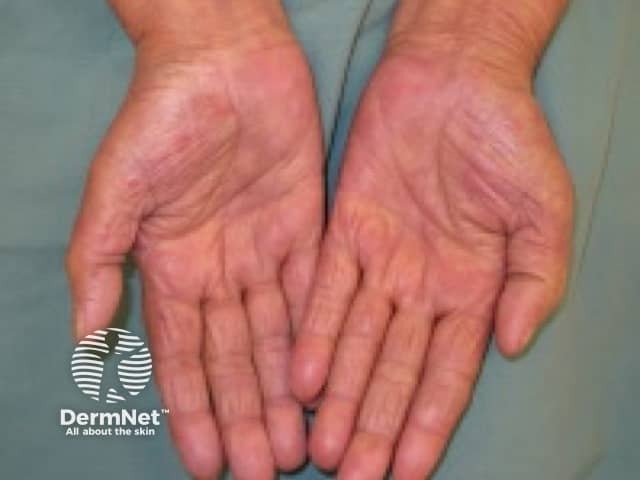
Hand-foot reaction
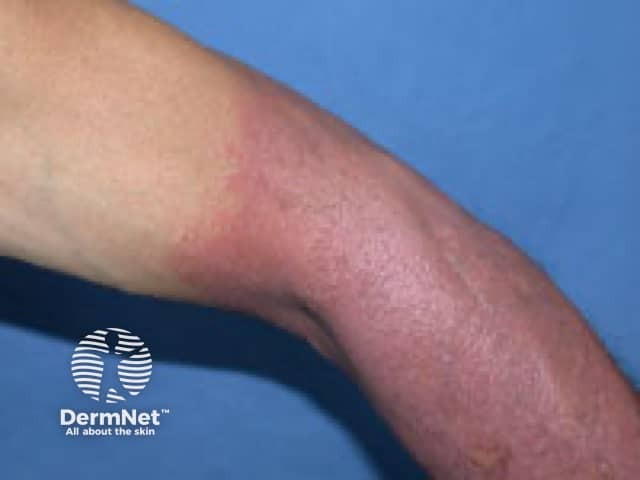
Photosensitivity
EGFR Inhibitors
Epidermal growth factor receptor (EGFR) signalling causes downstream activation of the heterodimeric lipid kinase phosphatidylinositol-3-kinase (PI3K) and AKT. Current therapies inhibiting the resulting cell proliferation include gefitinib, erlotinib, icotinib, dacomitinib, afatinib, osimertinib, poziotinib, mobocertinib, alpelisib, ipatasertib, capivasertib, and uprosertib.
The most common dermatologic toxicities are:
- Alopecia
- Hand-foot reaction
- Nail changes
- Pruritus
- Stomatitis
- Cheilitis
- Paronychia
- Acneiform rash
- Maculopapular rash.
For more information, see cutaneous side effects of EGFR and protein kinase inhibitors.
FGFR Inhibitors
Alterations in the fibroblast growth factor receptor (FGFR) family of tyrosine kinases have been linked to ~7% of all human cancers. Therapies targeting FGFR include erdafitinib, rogaratinib, futibatinib, infigratinib, pemigatinib, derazantinib, Debio 1347, and isogatinib.
Adverse cutaneous reactions include:
- Stomatitis
- Alopecia
- Hand-foot reaction
- Dry mouth
- Xerosis
- Nail changes or discoloration
- Calcinosis cutis.
Cell Division and DNA Repair
Hallmarks of cancer associated with cell division include genomic instability, evading growth suppression, and replicative immortality. Common anticancer therapeutic targets associated with cell division and DNA repair include PARP and CDK4/6.
PARP Inhibitors
The poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) enzyme is involved in DNA repair via nucleotide and base excision repair. Therapies targeting PARP include olaparib, rucaparib, talazoparib, niraparib, and veliparib.
Adverse cutaneous reactions include:
CDK4/6 Inhibitors
The G1 to S phase transition of the cell cycle is driven by cyclin-dependent kinases 4 and 6 (CDK4/6). Current anticancer therapies include abemaciclib, ribociclib, and palbociclib.
Dermatologic toxicities associated with CDK4/6 inhibitors are rare. A combination trial with aromatase inhibitor therapy reported a higher rate of rash, alopecia, pruritus, and stomatitis in the CDK4/6 and aromatase inhibitor group compared to placebo.
Myeloproliferative Neoplasms
Targeted cancer therapy for myeloproliferative disorders utilize BCR-ABL inhibitors and Janus kinase inhibitors.
BCR-ABL Inhibitors
Chronic myeloid leukaemia (CML) is a myeloproliferative neoplasm, resulting from the Philadelphia (Ph) chromosome balanced translocation, associated with the BCR-ABL fusion oncoprotein. Imatinib is a current therapy that targets BCR-ABL.
Cutaneous adverse reactions include:
- Maculopapular rash
- Lichenoid rash
- Erythroderma/exfoliative dermatitis
- Pityriasis rosea-like eruptions
- Psoriasiform dermatitis
- Psoriasiform palmoplantar hyperkeratosis with nail dystrophy
- Immunotherapy-related psoriasiform reactions
- Alopecia
- Photosensitivity
- Hair changes
- Melanocyte changes
- Pruritus
- Xerosis.
Janus Kinase Inhibitors
Primary myelofibrosis and polycythemia vera are frequently associated with mutation of the Janus kinase 2 (JAK2) non-receptor tyrosine kinase (V617F). Ruxolitinib is a current anticancer Janus kinase inhibitor.
Adverse cutaneous reactions include:
- Basal cell carcinoma
- Squamous cell carcinoma
- Pruritus
- Ecchymoses
- Herpes zoster infection
- Interstitial granulomatous drug eruptions
- Sweet syndrome
- Erythematous skin lesions with necrotic foci.
Bibliography
- Anforth R, Fernandez-Peñas P, Long GV. Cutaneous toxicities of RAF inhibitors. Lancet Oncol. 2013;14(1):e11–e18. doi:10.1016/S1470-2045(12)70413-8. Journal
- Autier J, Escudier B, Wechsler J, Spatz A, Robert C. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144(7):886–92. doi:10.1001/archderm.144.7.886. Journal
- Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395(10229):1078–88. doi:10.1016/S0140-6736(20)30164-1. Journal
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):782–9. doi:10.1016/S1470-2045(12)70269-3 Journal
- Ibrahim EM, Kazkaz GA, Abouelkhair KM, Bayer AM, Elmasri OA. Sunitinib adverse events in metastatic renal cell carcinoma: a meta-analysis. Int J Clin Oncol. 2013;18(6):1060–9. doi:10.1007/s10147-012-0497-2. Journal
- Kaul S, Kaffenberger BH, Choi JN, Kwatra SG. Cutaneous Adverse Reactions of Anticancer Agents. Dermatol Clin. 2019;37(4):555–68. doi:10.1016/j.det.2019.05.013. Journal
- Seervai RNH, Cho WC, Chu EY, et al. Diverse landscape of dermatologic toxicities from small-molecule inhibitor cancer therapy. J Cutan Pathol. 2022;49(1):61–81. doi:10.1111/cup.14145. Journal
- Shi VJ, Levy LL, Choi JN. Cutaneous manifestations of nontargeted and targeted chemotherapies. Semin Oncol. 2016;43(3):419–25. doi:10.1053/j.seminoncol.2016.02.018. Journal
On DermNet
- Cutaneous side effects of EGFR and protein kinase inhibitors
- Cutaneous adverse effects of checkpoint inhibitors
- Cutaneous adverse effects of antimelanoma therapies
- Cutaneous adverse effects of targeted anticancer therapies
- Skin toxicity of chemotherapy drugs
- Targeted cancer therapies
