Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Ruxolitinib — extra information
Ruxolitinib
Author(s): Dr Libby Whittaker, Medical Writer, DermNet; William Ju, University of Otago, New Zealand (2023)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction Uses How it works Precautions and contraindications Use in specific populations Benefits Disadvantages Side effects and risks
What is ruxolitinib?
Ruxolitinib is a selective Janus-activated tyrosine kinases inhibitor (JAK1 and JAK2), available in oral (Jakafi™) and topical (Opzelura™) formulations.
It is used to treat myelofibrosis, graft-versus-host disease, and in dermatology, atopic dermatitis, and non-segmental vitiligo.
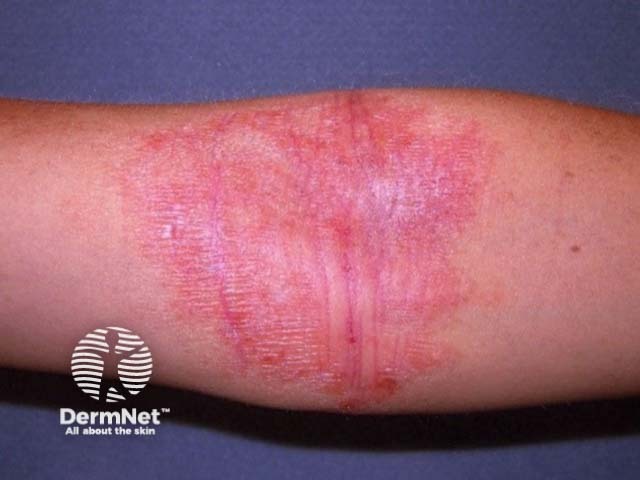
Antecubital fossa atopic eczema suitable for ruxolitinib cream
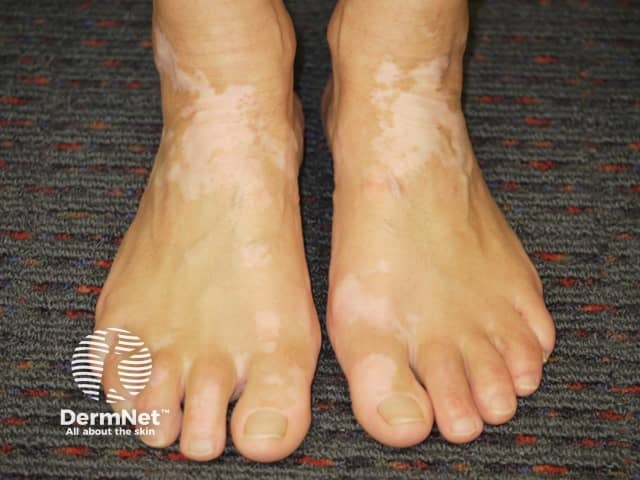
Ankle vitiligo suitable for ruxolitinib cream
What is ruxolitinib used for?
Oral ruxolitinib is approved for the treatment of:
- Intermediate or high-risk myelofibrosis (primary or due to polycythaemia vera or essential thrombocythemia)
- Steroid-refractory acute and chronic graft-versus-host disease (GVHD) in patients aged 12 years and older.
Topical ruxolitinib is approved for the treatment of the following conditions in adults and children ≥12 years old:
- Mild-to-moderate atopic dermatitis (eczema)
- Non-segmental vitiligo.
Other potential uses currently under investigation include:
How does ruxolitinib work?
Janus kinase (JAK) 1 and 2 mediate the signalling of cytokines and growth factors which are important for the process of haematopoiesis and immune function. Janus kinase inhibitors downregulate these inflammatory cytokines, which can be a useful mechanism of action in a variety of immune-mediated conditions including graft-versus-host disease, atopic dermatitis, and vitiligo.
What are the precautions and contraindications with ruxolitinib?
Contraindications
- Hypersensitivity reactions to ruxolitinib or any of its excipients.
- Avoid use in patients with active serious infections.
Precautions for ruxolitinib
- History of serious, recurrent, or opportunistic infections, or tuberculosis exposure (due to risk of infection).
- Patients who are current or ex-smokers (due to malignancy and cardiovascular risk).
- Recommended not to breastfeed while using.
Other precautions for oral ruxolitinib:
- Pre-treatment blood tests are recommended: full blood count, renal, and liver function tests.
- Concurrent use with strong CYP3A4 inhibitors (eg, grapefruit juice, azole antifungals, erythromycin, imatinib).
- More frequent blood tests recommended.
- Pregnancy Category C: adverse developmental outcomes associated with high maternal doses in rat and rabbit studies.
- Avoid live vaccines during use. Immunisations should be current before use. See also: Immunisation in immunosuppressed dermatology patients.
Other precautions for topical ruxolitinib:
- Do not apply to >10% (vitiligo) or >20% (atopic dermatitis) of body surface area (BSA).
- Do not use >60 g weekly or >100g fortnightly.
- Not for oral, ophthalmic, or vaginal use.
- Concurrent use with other Janus kinase inhibitors, biological treatments, or immunosuppressants (eg, ciclosporin, azathioprine) is not recommended.
- Pregnancy risks uncertain.
Dosing of ruxolitinib
Recommended starting dose:
- Acute graft-versus-host disease: 5 mg oral twice daily
- Chronic graft-versus-host: 10 mg oral twice daily.
After six months of treatment, tapering can be trialled every 8 weeks from 10 mg oral twice daily, to 5 mg twice daily, to 5mg once daily.
Topical ruxolitinib is available as 1.5% cream. Recommended dosing:
- Atopic dermatitis: thin layer twice daily on affected areas (<20% BSA)
- Vitiligo: thin layer twice daily on affected areas (<10% BSA).
Use in specific populations
Table 1. Dermatological use and dosing of ruxolitinib in specific populations
Population |
Recommendation |
|
Pregnancy |
|
|
Breastfeeding |
|
|
Paediatric |
|
|
Geriatric |
|
|
Renal impairment |
|
|
Hepatic impairment |
|
|
What are the benefits of ruxolitinib?
Oral ruxolitinib
Table 2. Evidence from REACH1-3 trials of oral ruxolitinib for steroid-refractory graft-versus-host disease (GVHD)*
Clinical trial |
Response |
Phase 2 trial for acute GVHD |
39/71 patients reached overall response after 28 days and 19 patients achieved complete response. |
Phase 3 trial for acute GVHD |
Overall response rate at 28 days was higher in the ruxolitinib group (96/154) than the control group (61/155). |
Phase 3 trial for chronic GVHD |
Overall response rate at 24 weeks was higher in the ruxolitinib group (82/165) than the control group which received best available other therapy (42/164). |
Topical ruxolitinib
- In phase 3 randomised, double-blind studies, improvement in Investigator’s Global Assessment (IGA) scores at week 8 was higher with ruxolitinib compared to vehicle (in > 12-year-old patients).
- Treatment success was achieved by 39–50% of the 0.75% ruxolitinib cream group, 51.3–53.8% of the 1.5% ruxolitinib cream group, and 7.6–15.1% of the vehicle group.
- Response was dose-related and higher rates of rapid itch reduction (within 12 hours of first application) were reported in the 1.5% ruxolitinib cream group.
- In a randomised phase 2 trial, ≥50% improvement in facial vitiligo area scoring index (F-VASI50) at week 24 was higher with ruxolitinib vs vehicle (in adult patients).
- F-VASI50 was achieved by 45–50% of those using 1.5% ruxolitinib cream once daily (n = 30) or twice daily (n = 33) vs 1/32 (3%) of the vehicle cream group.
What are the disadvantages of ruxolitinib?
- Monitoring requirements including periodic blood tests (every 1–2 weeks until dose of oral ruxolitinib stabilised) and clinical review including history, examination, and skin checks for any evidence of infection or malignancy.
What are the side effects and risks of ruxolitinib?
Adverse effects are more common for oral than topical ruxolitinib, and may include:
- Haematological (dose-related): thrombocytopaenia, anaemia, and neutropaenia.
- Infections (viral, bacterial, mycobacterial, or fungal), such as:
- Cytomegalovirus
- Skin infection eg, folliculitis
- Herpes zoster
- Ear infection
- Nasopharyngitis, bronchitis, or pneumonia
- Tuberculosis
- Urinary tract infection
- Sepsis.
- Gastrointestinal:
- Nausea and vomiting, flatulence, diarrhoea, or constipation
- Deranged liver enzyme levels, most frequently GGT, ALT, or AST
- Elevated lipase.
- Metabolic and cardiovascular:
- Hypercholesterolaemia
- Weight gain
- Hypertension.
- Renal: increased serum creatinine.
- Other:
Other serious potential adverse effects may include:
- Non-melanoma skin cancers (eg, basal cell carcinoma, squamous cell carcinoma, or Merkel cell carcinoma)
- Progressive multifocal leukoencephalopathy, a rare but serious viral infection affecting the central nervous system.
Higher rates of other malignancies (eg, lymphoma), thromboembolic events (eg, deep vein thrombosis, pulmonary embolism), and other adverse cardiovascular events (eg, myocardial infarction or stroke) were observed in a study comparing another Janus kinase inhibitor, tofacitinib, to tumour necrosis factor inhibitors for rheumatoid arthritis.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
Bibliography
- Brumfiel CM, Patel MH, Severson KJ, et al. Ruxolitinib Cream in the Treatment of Cutaneous Lichen Planus: A Prospective, Open-Label Study. J Invest Dermatol. 2022;142(8):2109–2116.e4. doi: 10.1016/j.jid.2022.01.015. Journal
- Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739–1749. doi: 10.1182/blood.2020004823 Journal
- Le RQ, Wang X, Zhang H, et al. FDA Approval Summary: Ruxolitinib for Treatment of Chronic Graft-Versus-Host Disease after Failure of One or Two Lines of Systemic Therapy. Oncologist. 2022;27(6):493–500. doi: 10.1093/oncolo/oyac042. Journal
- Mackay-Wiggan J, Jabbari A, Nguyen N, et al. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. 2016;1(15):e89790. doi: 10.1172/jci.insight.89790. Journal
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4):863–872. doi: 10.1016/j.jaad.2021.04.085. Journal
- Paudyal A, Zheng M, Lyu L, et al. JAK-inhibitors for dermatomyositis: A concise literature review. Dermatol Ther. 2021;34(3):e14939. doi: 10.1111/dth.14939. Journal
- Plosker GL. Ruxolitinib: A Review of Its Use in Patients with Myelofibrosis. Drugs. 2015;75(3):297–308. doi: 10.1007/s40265-015-0351-8. Journal
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396(10244):110–120. doi: 10.1016/S0140-6736(20)30609-7. Journal
- Sluczanowska-Glabowska S, Ziegler-Krawczyk A, Szumilas K, et al. Role of Janus Kinase Inhibitors in Therapy of Psoriasis. J Clin Med. 2021;10(19):4307. doi: 10.3390/jcm10194307. Journal
- Venetsanopoulou AI, Voulgari PV, Drosos AA. Janus kinase versus TNF inhibitors: where we stand today in rheumatoid arthritis. Expert Rev Clin Immunol. 2022;18(5):485–493. doi: 10.1080/1744666X.2022.2064275. Journal
- Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. N Engl J Med. 2021;385(3):228–238. doi: 10.1056/NEJMoa2033122. Journal
On DermNet
- Janus kinase inhibitors
- Immunisation in immunosuppressed dermatology patients
- Monitoring immune-modulating drugs used in dermatology
- Graft-versus-host disease
- Atopic dermatitis
- Vitiligo
Other websites
- Jakafi (oral ruxolitinib) prescribing information
- Opzelura (topical ruxolitinib) prescribing information
