Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Janus kinase inhibitors — extra information
Janus kinase inhibitors
Author: Dr Estella Janz-Robinson, Dermatology Registrar, Canberra Hospital, Canberra, Australia. Copy edited by Gus Mitchell. August 2021. Further updated by Hana Numan, Staff writer, December 2022 and June 2023.
Introduction
Uses
Contraindications
More information
Benefits
Disadvantages
Side effects and risks
What are Janus kinase inhibitors?
Janus kinase (JAK) inhibitors, also called jakinibs, are small molecules that interrupt the JAK-STAT (Signal Transducer and Activator of Transcription) signalling pathways involved in the pathogenesis of many immune-mediated and/or inflammatory diseases.
What are Janus kinase inhibitors used for?
Janus kinase inhibitors have shown beneficial effects in a variety of immune-mediated conditions affecting the skin, joints, and gastrointestinal tract. JAK inhibitors have also been used successfully in conditions with JAK mutations such as polycythaemia vera, essential thrombocythaemia, and myelofibrosis.
Tofacitinib received FDA approval in the US for the treatment of rheumatoid arthritis in 2012, and in 2017/18 also for psoriatic arthritis and ulcerative colitis. It is used off-label for psoriasis, and is currently under investigation for the treatment of several dermatological conditions including atopic dermatitis and vitiligo.
Ruxolitinib was approved by the TGA in Australia in 2013 for the treatment of myelofibrosis. It also became FDA-approved for the topical treatment of mild to moderate atopic dermatitis (2021), and non-segmental vitiligo in adult and paediatric patients 12 years of age and older (2022).
Baricitinib was TGA-approved in Australia in 2018 for the treatment of rheumatoid arthritis and, in 2021, for moderate to severe atopic dermatitis. Approval for this indication has also been granted by the European Union and Japan. Upadacitinib was approved by Medsafe New Zealand (2021) and by the FDA (2022) for the treatment of moderate to severe atopic dermatitis. Oral abrocitinib and upadacitinib received licensing for use in atopic eczema in the USA and Europe in 2023.
In 2020, delgocitinib cream was approved in Japan for the treatment of atopic dermatitis in adults, and was granted an FDA fast-track designation for the treatment of chronic hand dermatitis.
Use of Janus kinase inhibitors in dermatology
Janus kinase inhibitors have been approved for use in or are currently under investigation for the treatment of:
-
Atopic dermatitis
- Topical — delgocitinib, ruxolitinib, tofacitinib
- Oral — baricitinib, tofacitinib, abrocitinib
-
- Oral — tofacitinib, ruxolitinib, baricitinib
-
- Topical — ruxolitinib, tofacitinib
- Oral — tofacitinib
-
- Topical — ruxolitinib, brepocitinib
- Oral — tofacitinib, baricitinib, peficitinib, deucravacitinib, filotinib, delgocitinib, itacitinib
- Oral tofacitinib for nail psoriasis
-
Other dermatoses
- Chronic hand dermatitis — topical delgocitinib
- Dermatomyositis — oral ruxolitinib, tofacitinib
- Graft versus host disease (GVHD) — oral ruxolitinib, baricitinib, itacitinib — as prophylaxis or treatment of acute or chronic GVHD
- Lichen planus/lichen planopilaris — topical ruxolitinib, oral tofacitinib
- Systemic lupus erythematosus/discoid lupus erythematosus — oral baricitinib, elsubrutinib, upadacitinib
- Granulomatous disorders including sarcoidosis, granuloma annulare, necrobiosis lipoidica — oral tofacitinib
Single case reports or small case series reporting response to JAK inhibitors include: chronic actinic dermatitis, drug hypersensitivity syndrome (DRESS), hypereosinophilic syndrome, morphoea, eosinophilic fasciitis, and hidradenitis suppurativa.
What are the contraindications with Janus kinase inhibitors?
- Hypersensitivity to the drug or any of the product excipients.
- Concurrent biologic agents or other potent immunosuppressant agents.
- Severe liver impairment.
- Pregnancy and lactation: Tofacitinib and baricitinib are rated Category D in Australia as teratogenic effects have been shown in animal studies. They are not recommended for use when breastfeeding. Ruxolitinib crosses the placenta and is excreted in breast milk, so is not recommended in pregnancy (Category C) and lactation.
Tell me more about Janus kinase inhibitors.
The Janus kinase family consists of four receptor-associated tyrosine kinases — JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). When activated, JAKs phosphorylate cell membrane cytokine receptors and intracytoplasmic STATs, thereby modulating gene transcription in the nucleus. The phosphate is provided by ATP, and each JAK and tyrosine kinase recognises a slightly different ATP-binding pocket. Overexpression of these pathways can result in the development of autoimmune disease and malignancies.
JAK inhibitors (JAKi) can be classified as:
- Pan-JAKi — delgocitinib, peficitinib
- JAK1i — oclacitinib, upadacitinib, abrocitinib, itacitinib
- JAK1/2i — baricitinib, ruxolitinib
- JAK1/3i — tofacitinib
- JAK3i — decernotinib, ritlecitinib
- TYK2 inhibitors — deucravacitinib, brepocitinib.
Janus kinase inhibitor signalling pathways
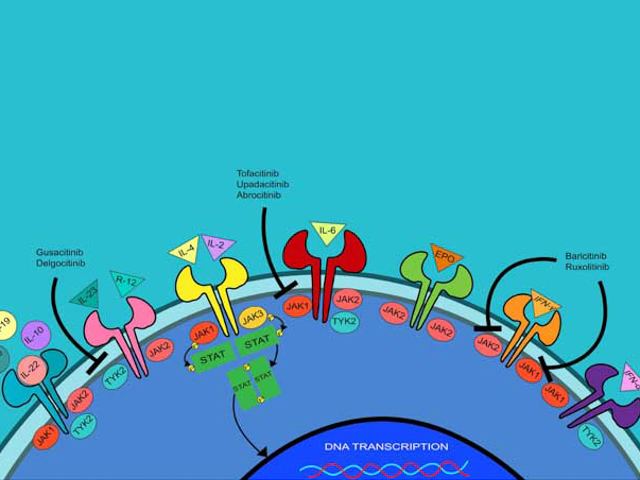
Image from: Singh R, Heron CE, Ghamrawi RI, Strowd LC, Feldman SR. Emerging role of Janus kinase inhibitors for the treatment of atopic dermatitis. Immunotargets Ther. 2020;9:255-72. doi:10.2147/ITT.S229667
What are the benefits of Janus kinase inhibitors?
As Janus kinase inhibitors are small molecules they can be used topically or orally, unlike biologic agents which require administration by injection. They are also significantly cheaper than biologics as JAK inhibitors are chemically synthesised.
What are the disadvantages of Janus kinase inhibitors?
Janus kinase inhibitors may interrupt the signal transduction of multiple cytokine receptors rather than target specific cytokines or cytokine receptors.
For a number of dermatological conditions, clinical trials have shown rapid relapse is common after discontinuation of the JAK inhibitor such as alopecia areata, vitiligo, and psoriasis.
What are the side effects and risks of Janus kinase inhibitors?
As Janus kinase inhibitors alter the immune response, they may be associated with increased risk of serious bacterial, fungal, mycobacterial, and viral infections. Tuberculosis screening should therefore be undertaken before starting treatment with a JAK inhibitor. Reactivation of herpes viruses appears to be common, therefore zoster vaccination prior to commencing treatment is recommended.
Common adverse side effects of JAK inhibitors include:
- Nasopharyngitis
- Infection of upper respiratory and urinary tracts
- Headache
- Nausea and diarrhoea
- Acne - mild acne occurs in about 15% of those with eczema treated with JAKis - it is usually responsive to topical agents such as benzoyl peroxide, antibiotics or retinoids, and seldom requires drug withdrawal.
Selective JAK inhibitors may show unique adverse effect profiles such as cytopenias linked to selective JAK2 inhibition.
Tofacitinib has been used long-term in patients with rheumatoid arthritis:
- Increased lipids may be seen during the first 3 months of treatment, then stabilise
- Malignancies are not increased except for non-melanoma skin cancers
- The TGA in Australia warns high dose (10mg bd) should be used with caution in patients with non-melanoma skin cancer, serious infections, and herpes zoster infection.
In 2021, the FDA issued new updated black box warnings for baricitinib and upadacitinib in line with warnings already in place for tofacitinib. Tofacitinib has been reported to carry increased risks of serious heart-related events, blood clots, and death even at low dose. Although similar adverse events have not yet been reported with baricitinib and upadacitinib, the FDA found the risks have not been adequately evaluated and issued the warning due to the similar mechanisms of action.
An association between JAK inhibitors and malignancy (eg, T-cell lymphomas) is currently under investigation and is not fully understood. There have been rare cases of T-cell lymphomas reported in patients treated with JAK inhibitors, but the overall incidence appears to be low and causality has not been established.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
Bibliography
- Chapman S, Kwa M, Gold LS, Lim HW. Janus kinase inhibitors in dermatology: Part I. A comprehensive review. J Am Acad Dermatol. 2021;S0190-9622(21)02071–5. doi:10.1016/j.jaad.2021.07.002. PubMed
- Chapman S, Gold LS, Lim HW. Janus kinase inhibitors in dermatology: Part II. A comprehensive review. J Am Acad Dermatol. 2021;S0190-9622(21)02022-3. doi:10.1016/j.jaad.2021.06.873. PubMed
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691–714. doi:10.2147/CCID.S309215. Journal
- Fragoulis GE, McInnes IB, Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology (Oxford). 2019;58(Suppl 1):i43–54. doi:10.1093/rheumatology/key276. Journal
- Jo CE, Gooderham M, Beecker J. TYK 2 inhibitors for the treatment of dermatologic conditions: the evolution of JAK inhibitors. Int J Dermatol. 2021;10.1111/ijd.15605. doi:10.1111/ijd.15605. PubMed
- Kołkowski K, Trzeciak M, Sokołowska-Wojdyło M. Safety and Danger Considerations of Novel Treatments for Atopic Dermatitis in Context of Primary Cutaneous Lymphomas. Int J Mol Sci. 2021;22(24):13388. Published 2021 Dec 13. doi:10.3390/ijms222413388 Journal
- Kandai Saito and others, A case of Sézary syndrome in a patient during treatment with baricitinib for seronegative rheumatoid arthritis, Clinical and Experimental Dermatology, Volume 48, Issue 4, April 2023. Journal
- Krueger JG, McInnes IB, Blauvelt A. Tyrosine kinase 2 and Janus kinase‒signal transducer and activator of transcription signaling and inhibition in plaque psoriasis. J Am Acad Dermatol. 2021;S0190-9622(21)02017-X. doi:10.1016/j.jaad.2021.06.869. Journal
- Lin CM, Cooles FA, Isaacs JD. Basic mechanisms of JAK inhibition. Mediterr J Rheumatol. 2020;31(Suppl 1):100–4. doi:10.31138/mjr.31.1.100. Journal
- Mendes-Bastos P, Ladizinski B, Guttman-Yassky E, et al. Characterization of acne associated with upadacitinib treatment in patients with moderate-to-severe atopic dermatitis: A post hoc integrated analysis of 3 phase 3 randomized, double-blind, placebo-controlled trials. J Am Acad Dermatol. 2022;87(4):784-791. Journal
- Singh R, Heron CE, Ghamrawi RI, Strowd LC, Feldman SR. Emerging role of Janus kinase inhibitors for the treatment of atopic dermatitis. Immunotargets Ther. 2020;9:255-72. doi:10.2147/ITT.S229667 Journal
- Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847. doi:10.3389/fimmu.2019.02847. Journal
- Worm M, Bauer A, Elsner P, Mahler V, Molin S, Nielsen TSS. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182(5):1103–10. doi:10.1111/bjd.18469. Journal
