Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Key clinical-trial evidence for cobimetinib — extra information
Key clinical-trial evidence for cobimetinib
Author: Anoma Ranaweera, Medical Writer, Auckland, Wellington. Chief Editor: DermNet New Zealand Editor in Chief: Hon A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy editor: Gus Mitchell/Maria McGivern, August 2017.
What is cobimetinib?
Cobimetinib is a prescription medicine used to treat melanoma that has spread to other parts of the body, cannot be removed by surgery, and has an abnormal BRAF gene. It should not be used to treat melanoma in patients with a normal or wild-type BRAF gene.
In 2015, the US Food and Drug Administration (FDA) approved cobimetinib (Cotellic™, Genentech Inc. California, USA) in combination with vemurafenib (a specific BRAF–kinase inhibitor) for the treatment of melanoma.
In the same year, the Committee for Medicinal Products for Human Use at the European Medicines Agency issued a positive opinion for marketing authorization for cobimetinib in the European Union.
Cobimetinib has since received approval for marketing in New Zealand through Medsafe in 2017 for the treatment of patients with melanoma.
FDA approval was based on results from the Phase III CoBRIM study, which showed cobimetinib, when used in combination with vemurafenib, reduced the risk of disease worsening or death by about half in patients with melanoma.
Key clinical trial evidence for cobimetinib
The CoBRIM study
CoBRIM was an international, randomised, double-blind, placebo-controlled Phase III study that evaluated the safety and efficacy of 60 mg of cobimetinib once daily plus 960 mg twice daily of vemurafenib compared to 960 mg of vemurafenib twice daily plus placebo. A total of 495 patients were involved in the study, all of whom had previously untreated BRAF V600 mutation-positive, unresectable, locally advanced or metastatic melanoma.
The presence of BRAF V600 mutation was detected using the cobas®; 407 4800 BRAF V600 mutation test.
- All patients received vemurafenib 960 mg orally twice daily on days 1–28.
- Patients were randomised to receive cobimetinib 60 mg or matching placebo orally once daily on days 1–21 of every 28-day cycle. Randomisation was stratified by geographic region (North America vs. Europe vs. Australia/New Zealand/others) and disease stage (unresectable Stage IIIc, M1a, or M1b vs. Stage M1c).
- Treatment was continued until disease progression, unacceptable toxicity, or withdrawal of consent.
- Patients randomised to receive placebo were not offered cobimetinib at the time of disease progression.
- The major efficacy outcome was investigator-assessed progression-free survival (PFS) per RECIST v1.1.
- Secondary endpoints included PFS by independent review committee, objective response rate, overall survival and duration of response.
Efficacy results are summarised in Table 1.
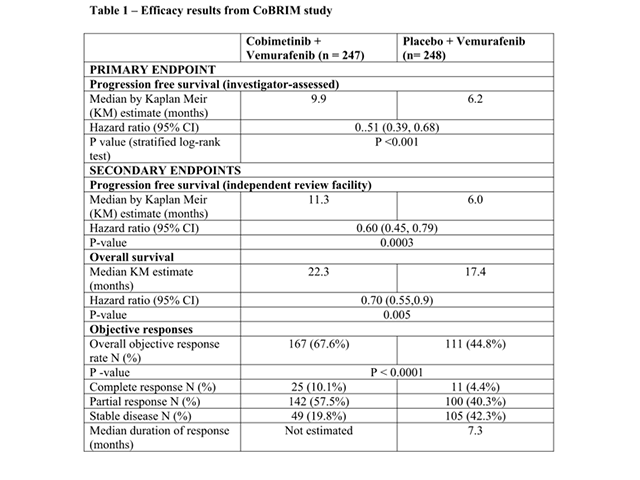
Efficacy results of CoBRIM study
Adverse events — clinical trial experience (CoBRIM study)
The most common (≥ 20%) adverse reactions with cobimetinib were:
- Diarrhoea
- Photosensitivity reaction
- Nausea
- Pyrexia
- Vomiting.
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in clinical trials may not reflect the rates observed in practice. In the CoBRIM study, 15% of patients receiving cobimetinib experienced an adverse reaction that resulted in permanent drug discontinuation.
The most common adverse reactions resulting in permanent drug discontinuation were:
- Liver laboratory abnormalities, defined as increased aspartate aminotransferase (2.4%), increased gamma glutamyltransferase (1.6%), and increased alanine aminotransferase (ALT; 1.6%)
- Rash (1.6%)
- Pyrexia (1.2%)
- Retinal detachment (2%).
Among the 247 patients receiving cobimetinib, adverse reactions led to dose interruption or reductions in 55%. The most common reasons for dose interruptions or reductions of cobimetinib were:
- Rash (11%)
- Diarrhoea (9%)
- Chorioretinopathy — fluid buildup under the retina (7%)
- Pyrexia (6%)
- Vomiting (6%)
- Nausea (5%)
- Increased creatine phosphokinase (4.9%).
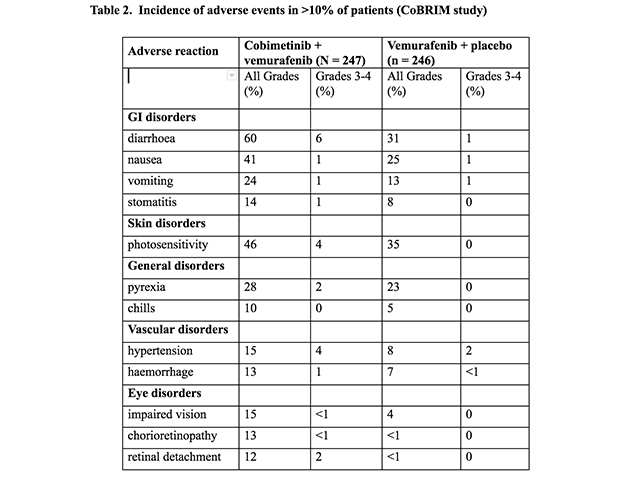
Adverse events (CoBRIM study)
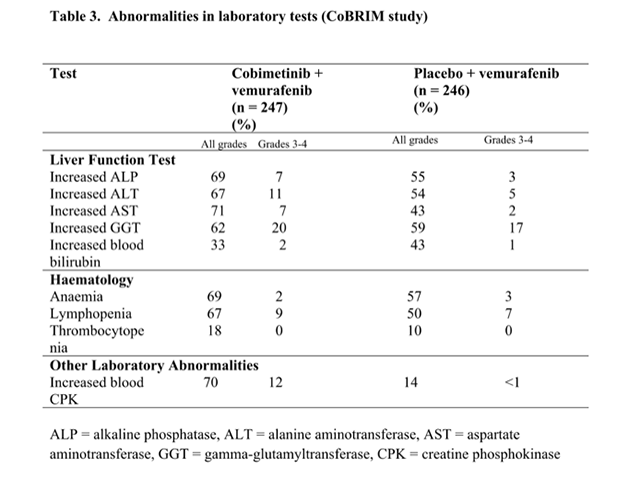
Abnormalities in laboratory tests (CoBRIM study)
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after the authorisation of a medicine is important, as it allows continued monitoring of the benefit/risk balance of the medicine. Healthcare professionals in New Zealand are advised to report any suspected adverse reactions to the New Zealand Pharmacovigilance Centre.
Overdose
There is no experience with cobimetinib overdose in human clinical trials. In case of suspected overdose, cobimetinib should be withheld and supportive care instituted. There is no specific antidote for overdosage with cobimetinib.
The New Zealand National Poisons Centre should be contacted on 0800 764 766 for advice on the management of overdose.
Cobimetinib — future potential
- New treatments for metastatic melanoma work through distinct mechanisms of enhancing the immune response and blocking cellular proliferation.
- Like trametinib, cobimetinib is a MEK inhibitor. MEK1 and MEK2 are tyrosine kinases that interact with BRAF and lead to uncontrolled growth of melanoma cells.
- Clinical trials have shown that the addition of cobimetinib to vemurafenib results in improvements in survival in patients with melanoma harbouring the V600 BRAF mutation.
- Overall survival in patients treated with nivolumab and pembrolizumab appears to be superior compared with that achieved with cobimetinib and vemurafenib.
- However, overall survival in patients treated with a combination of vemurafenib and cobimetinib appears to be similar to other available treatment strategies for the treatment of unresectable metastatic malignant melanoma such as combinations of the nivolumab and ipilimumab and dabrafenib with trametinib.
- For PFS, dabrafenib in combination with trametinib, and vemurafenib combined with cobimetinib, seemed to have a higher probability of good performance than other available treatment strategies.
- For serious adverse events, pembrolizumab and nivolumab seemed to have a higher probability of fewer serious adverse events than cobimetinib and vemurafenib.
- However, none of these interventions are cost-effective at maximum pharmacy retail prices, and the budgetary impacts in clinical practice can be substantial.
- The rapid tumour response to BRAF/MEK inhibition is often short-lived as tumours develop resistance to combination therapy.
- New trials are beginning to investigate the addition of a third targeted agent or immunotherapy in order to increase the durability of treatment response.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371: 1867–76. DOI: 10.1056/NEJMoa1408868. Journal
- Ribas A, Gonzalez R, Pavlick A, et al. Combination of vemurafenib and cobimetinib in patients with advanced BRAF (V600)-mutated melanoma: a phase 1b study. Lancet Onco. 2014; 15: 954–65. DOI: 10.1016/S1470-2045(14)70301-8. Pubmed
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF (V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 2016; 17: 1248–60. DOI: 10.1016/S1470-2045(16)30122-X. Pubmed
- Cobimetinib (Cotellic) for metastatic melanoma. The Medical Letter on Drugs and Therapeutics 2016; 58 (1491): 434. Journal
- Signorelli J, Shah Gandhi A. Cobimetinib. Ann Pharmacother 2017; 51: 146–153. DOI: 10.1177/1060028016672037. Pubmed
On DermNet
Other websites
- Cotellic (cobimetinib) tablets, for oral use — US FDA prescribing Information
- Cobimetinib 20 mg film-coated tablets — Medsafe New Zealand data sheet
- Melanoma: assessment and management — Nice Guideline [NG14] July 2015
- New Zealand National Poisons Centre
- New Zealand Pharmacovigilance Centre
