Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Quinacrine — extra information
Quinacrine
Authors: Dr Anes Yang, Clinical Researcher, Department of Dermatology, St George Hospital, University of New South Wales, Sydney, NSW, Australia; Dr Monisha Gupta, Senior Staff Specialist, Department of Dermatology, Liverpool Hospital, Sydney, NSW, Australia. Dr Delwyn Dyall-Smith, Dermatologist, Wagga Wagga, NSW, Australia. Adjunct Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. September 2018.
Introduction Uses How it works Side effects and risks Contraindications
What is quinacrine?
Quinacrine is a derivative of quinine, synthesised from the bark of the cinchona tree. It was extensively used as an antimalarial agent for millions of servicemen in the Pacific region during the 1940s. It was found to be well tolerated and has subsequently been used for the treatment of connective tissue diseases, such as lupus erythematosus. However, due to yellowish skin changes that occur after long-term use, it is not used as commonly as hydroxychloroquine or chloroquine [1,2].
Quinacrine is also known as mepacrine and has the brand name Atabrine™.
Who uses quinacrine?
The use of quinacrine in dermatology has been mostly superseded by chloroquine and hydroxychloroquine [2]. Although it can be used alone, quinacrine is usually used in combination with hydroxychloroquine to boost response in recalcitrant inflammatory skin conditions such as:
Quinacrine may be preferred in patients at ocular risk or those with profound fatigue as it is a central nervous system stimulant [1]. It is also useful in those who are unable to tolerate chloroquine derivatives.
Skin conditions for which quinacrine is used
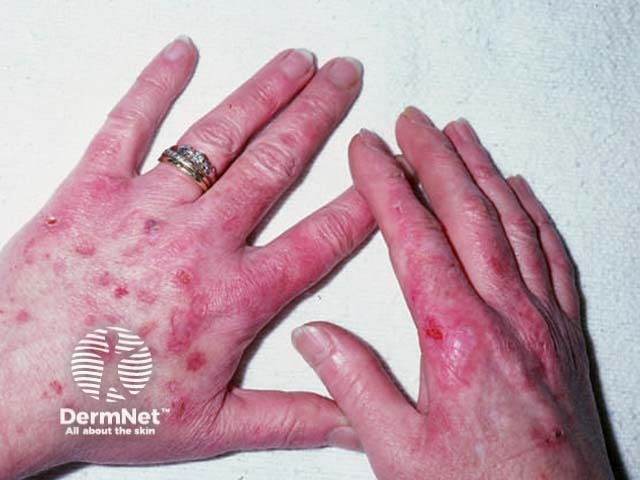
Discoid lupus erythematosus
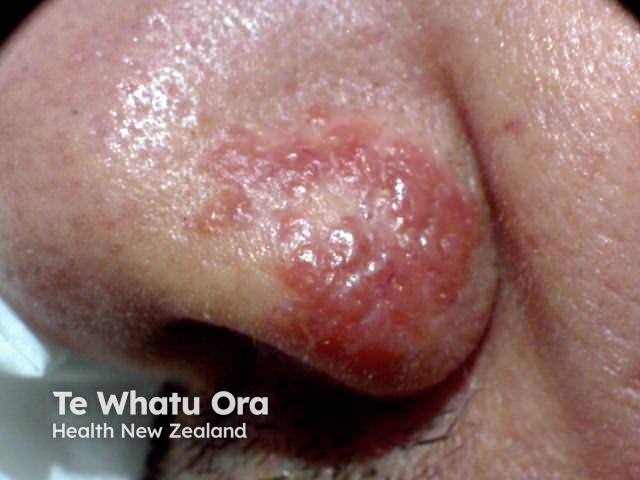
Lupus pernio
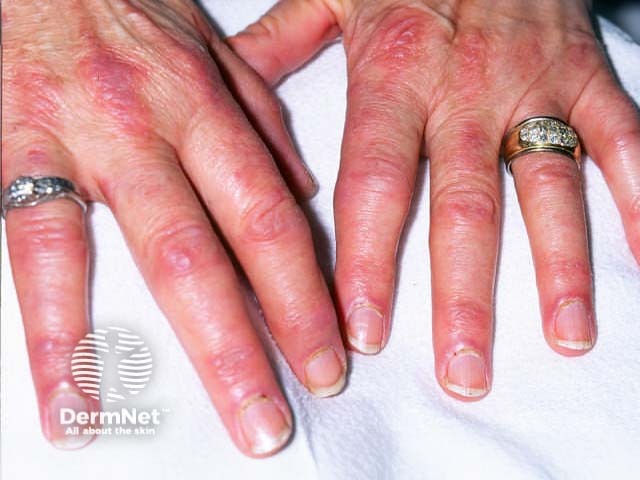
Dermatomyositis
How does quinacrine work?
Quinacrine has been shown to have the following effects.
- Antiproliferative
- Anti-inflammatory
- Antiparasitic
- Anticancer effects [3].
Pharmacokinetics
Quinacrine is rapidly absorbed from the gastrointestinal tract following oral administration. The plasma concentration of quinacrine increases rapidly during the first week and equilibrates by the fourth week. Quinacrine is slowly excreted, mainly via the kidneys [1,3].
Dosing
Quinacrine is dispensed as 100 mg tablets. The recommended maintenance dose is 100 mg orally daily, or on alternate days in fair-skinned patients due to possible skin discolouration. The onset of action is 3–6 weeks, depending on the initial dose. If there has been no improvement after 8 weeks, then the quinacrine should be ceased [1].
Quinacrine is often combined with hydroxychloroquine, when hydroxychloroquine alone is ineffective (eg, hydroxychloroquine 200–400 mg daily with 50–100 mg quinacrine every second day).
Quinacrine is an older medication and is no longer marketed in Australia but is available through the Australian Therapeutic Goods Administration's Special Access Scheme. It is not available in New Zealand.
What are the side effects and risks of quinacrine?
Quinacrine is generally well tolerated. Common minor adverse effects such as transient headache, gastrointestinal symptoms (eg, nausea, abdominal cramping, and diarrhoea), and dizziness, improve with time or a reduction in the dose [1,3].
Quinacrine commonly causes yellowing and bruise-like discolouration of the skin, oral mucosa, nails, and sclera that is not related to liver injury. This discolouration is more prominent in fair-skinned patients but is harmless and will fade when the treatment is stopped [1,3]. Dermatitis is another common skin complaint that resolves with the discontinuation of quinacrine [1].
Unlike chloroquine or hydroxychloroquine, quinacrine has a very low risk of retinal toxicity [4].
High doses of quinacrine, usually exceeding those used in dermatology, can cause more serious side effects. These side effects include corneal oedema (> 500 mg/days), neurological issues (1200 mg/days), liver toxicity, and aplastic anaemia [3]. Although the latter has rarely been reported in doses of quinacrine < 300 mg/day, routine blood test monitoring is recommended. The aplastic anaemia is often preceded by a lichen planus-like rash.
Pregnancy and antimalarial medications
Quinacrine crosses into the placenta; therefore, should not be given to pregnant women, or women planning a pregnancy (see DermNet's pages on Safety of medicines taken during pregnancy and on Lactation and medications used in dermatology).
Quinacrine is excreted in small amounts in breast milk. Although problems have not been reported, its use during breastfeeding should be avoided if possible.
Children and elderly patients
Children tolerate quinacrine less well than adults and it may cause vomiting due to its bitter taste.
Appropriate studies in elderly populations have not been performed for quinacrine; however, no geriatric-specific problems have been documented.
What are the contraindications with quinacrine?
Women planning a pregnancy, and pregnant and lactating women should not take quinacrine.
Caution should be taken in:
- The use of quinacrine in children under 5 years of age
- Excessive alcohol consumption
- Patients with severe liver disease.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Wallace DJ. The use of quinacrine (Atabrine) in rheumatic diseases: a reexamination. Semin Arthritis Rheum 1989; 18: 282–96. PubMed
- Benoit S, Goebeler M. Mepacrine in recalcitrant cutaneous lupus erythematosus: Old-fashioned or still useful? Acta Derm Venereol 2015; 95: 596–9. DOI: 10.2340/00015555-2031. PubMed
- Ehsanian R, Van Waes C, Feller SM. Beyond DNA binding — a review of the potential mechanisms mediating quinacrine's therapeutic activities in parasitic infections, inflammation, and cancers. Cell Commun Signal 2011; 9: 13. DOI: 10.1186/1478-811X-9-13. PubMed Central
- Zuehlke RL, Lillis PJ, Tice A. Antimalarial therapy for lupus erythematosus: an apparent advantage of quinacrine. Int J Dermatol 1981; 20: 57–61. PubMed
On DermNet
- Hydroxychloroquine
- Chloroquine
- Antimalarial medications in dermatology
- Oral medications for skin diseases
- Cutaneous lupus erythematosus
- Sarcoidosis
- Dermatomyositis
Other websites
- Recommendations on screening for chloroquine and hydroxychloroquine retinopathy — 2016 — American Academy of Ophthalmology
- Mepacrine — British Association of Dermatologists
- Special Access Scheme — Australian Therapeutic Goods Administration
