Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Chloroquine — extra information
Chloroquine
Authors: Dr Anes Yang, Clinical Researcher, Department of Dermatology, St George Hospital, University of New South Wales, Sydney, NSW, Australia; Dr Monisha Gupta, Senior Staff Specialist, Department of Dermatology, Liverpool Hospital, Sydney, NSW, Australia. DermNet Medical Editor: Dr Delwyn Dyall-Smith, Dermatologist, Wagga Wagga, NSW, Australia; Adjunct Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. September 2018.
Introduction
Uses
Contraindications
More information
Chloroquine dosing
Ocular side effects and risks
Other side effects
Drug interactions
Monitoring
What is chloroquine?
Chloroquine is a synthetic antimalarial medication that was first developed in 1934. In addition to its antimalarial effects, chloroquine has been used extensively in rheumatology and dermatology since the 1950s.
Despite its early successes, the use of chloroquine has mostly been superseded by hydroxychloroquine, a safer hydroxylated analogue, and its availability is now limited.
Chloroquine has immunomodulatory and anti-inflammatory effects and may also have a photoprotective effect [1].
Who uses chloroquine?
Chloroquine and hydroxychloroquine have the same indications and usage, although the dosage regimens are different. Head-to-head comparisons suggest similar responses with equivalent doses, but greater retinal toxicity with chloroquine [2].
In dermatology, antimalarial drugs, such as chloroquine, are regarded as appropriate treatments for:
- Lupus erythematosus
- Reticular erythematous mucinosis
- Porphyria cutanea tarda
- Chronic ulcerative stomatitis
- Sarcoidosis
- Dermatomyositis.
Antimalarial drugs are sometimes also used to treat other inflammatory skin diseases [3].
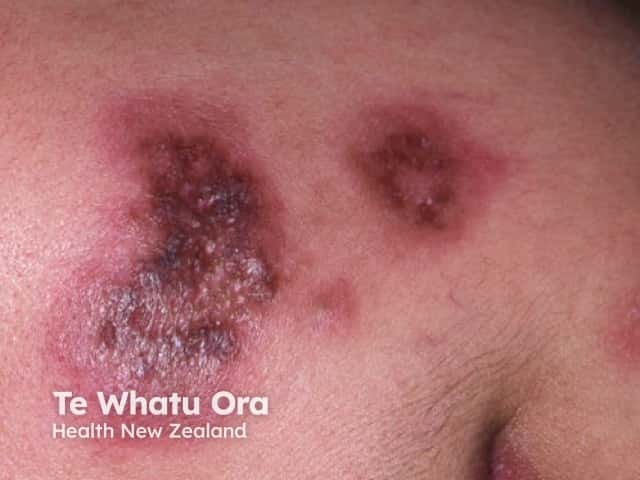
Discoid lupus erythematosus
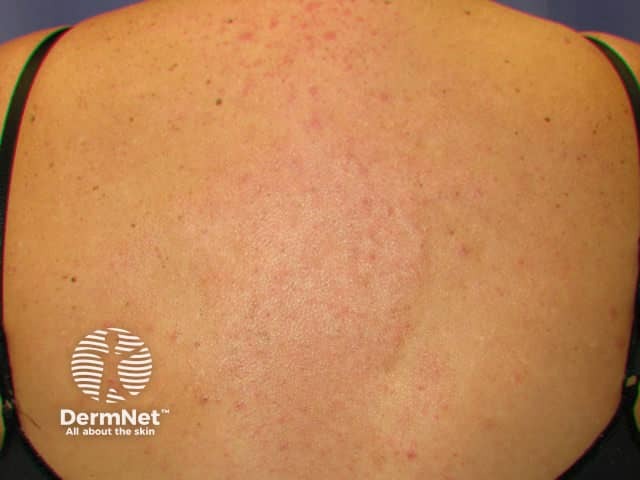
Reticular erythematous mucinosis
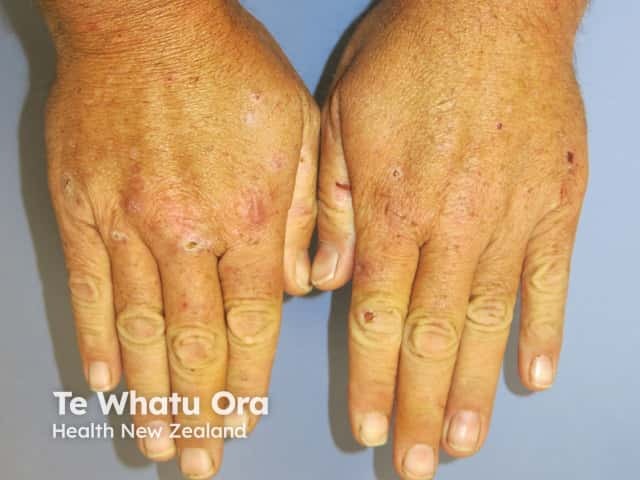
Porphyria cutanea tarda
What are the contraindications with chloroquine?
Children aged under 6 years of age should not be given chloroquine due to the risk of overdose and acute toxicity.
The chloroquine dose should be reduced in patients with renal disease, as up to 70% of chloroquine is excreted unchanged in the urine. Chloroquine itself can cause reduced kidney function of up to 10% of patients, especially in those over 60 years of age. Renal impairment results in higher blood levels of chloroquine and therefore an increased toxicity risk.
Chloroquine should be used with caution in patients with known porphyria cutanea tarda. Chloroquine can be used to treat porphyria cutanea tarda, but in a very low dose (125 mg twice weekly) as a dosage of 250 mg/day can trigger a porphyria crisis, which can be fatal.
Chloroquine is contraindicated where there is a known hypersensitivity to 4-aminoquinoline compounds.
Chloroquine crosses the placenta and is also found in low levels in breast milk, so pregnancy and lactation are often listed as contraindications to its use (see DermNet’s pages on Safety of medicines taken during pregnancy and Lactation and medications used in dermatology. However, effects on the fetus and baby have been rarely reported and chloroquine has been continued during pregnancy and lactation when required for malaria.
Pre-existing retinopathy (damage to the retina) and other eye problems, such as macular degeneration (blurred vision in the centre of the eye) and cataracts, make monitoring more difficult, and so can also be relative contraindications.
Although chloroquine can occasionally trigger a flare of psoriasis, this is not an absolute contraindication, and the use of the drug should be decided in individual cases.
Tell me more about chloroquine
Chloroquine has similar pharmacokinetics to hydroxychloroquine. After identical doses, the tissue levels of chloroquine are 2.5 times those seen with hydroxychloroquine. Chloroquine is rapidly and almost completely absorbed from the bowel following oral administration.
Peak plasma concentrations of chloroquine are reached within 4–12 hours, but it takes 4–6 weeks for plasma concentrations to stabilise; therefore, it will take 2–3 months to see a therapeutic effect. Up to 70% of chloroquine is excreted unchanged through the kidneys and up to 40% is metabolised in the liver into an active metabolite.
Chloroquine binds to plasma proteins that are deposited in tissues, such as the liver, spleen, kidney, and lung. Chloroquine has a particularly high affinity for melanin-containing cells and hence there are very high levels of chloroquine in the skin (mainly, the epidermis) and retina. Chloroquine has a long half-life, which can vary between 74 hours and 50 days, depending on the cumulative dose.
The drug can remain in the skin for 6–7 months after the cessation of therapy.
Chloroquine dosing
Chloroquine phosphate is dispensed as a 250 mg tablet, which is equivalent to 155 mg chloroquine base.
Long-term chloroquine use should aim for <2.3 mg/kg/day based on the patient's actual body weight. Given the tablet size, this usually means it needs to be used intermittently; that is, several times per week instead of daily. Previously, the maximum daily dose was calculated on the patient's ideal body weight, and cumulative dose was recommended to be less than 460 g. These guidelines have been revised.
In porphyria cutanea tarda, the dose should not exceed 125 mg twice weekly.
Chloroquine can be used together with quinacrine, but it should not be used with hydroxychloroquine.
Chloroquine is not marketed in Australia, where it is only available through the Therapeutic Goods Administration's Special Access Scheme. It is no longer approved for use in New Zealand, but may be available under Section 29 (see Medsafe's information on unapproved medications).
Dosing for hepatic impairment
No dosage adjustment of chloroquine for hepatic impairment is provided in the manufacturer’s labelling.
Dosing for renal impairment
The following guidance is given for chloroquine dosing in cases of renal impairment:
- Creatinine clearance ≥10 mL/minute: no dosage adjustment is necessary
- Creatinine clearance <10 mL/minute: administer 50% of usual dose.
What are the ocular side effects and risks of chloroquine?
The two main ocular effects of chloroquine are reversible corneal deposits and irreversible retinal toxicity.
Corneal deposits
Corneal deposits occur rapidly in 90% of patients on chloroquine. They are usually asymptomatic; however, patients can experience transient halos and heightened light sensitivity.
Finding corneal deposits on examination does not mean chloroquine should be stopped.
Deposits are dose related, occurring 1–1.5 months after therapy is initiated and resolve after the discontinuation of chloroquine.
Retinal toxicity
Irreversible retinal toxicity, causing bilateral bull’s eye retinopathy from chloroquine has been recognised for many years. This is a late-stage finding and should not be seen if regular monitoring is conducted. The high affinity of chloroquine for melanin-containing cells, such as those found in the retinal pigment epithelium, is hypothesised to be the cause.
Patients with antimalarial-induced retinal toxicity may not have any visual symptoms initially and will only develop clinical symptoms in the late stages.
The cessation of the drug when definite signs of early retinopathy are found is important, as chances of vision loss will typically decrease. Chloroquine should only be ceased if there are definite signs of retinal toxicity; possible toxicity should be reassessed after several months with continued drug use. Continued use of chloroquine after a finding of definite retinopathy results in irreversible visual damage, including effects on visual acuity, peripheral vision, and night vision.
This retinopathy does not reverse, even with the cessation of the chloroquine, and there is no treatment. The risk of retinal toxicity is highest with chloroquine compared to other antimalarials [4].
In 2016, with new scientific data, the American Academy of Ophthalmology revised their screening recommendations for patients on chloroquine for longer than 1 year [5]. The two most important risk factors for retinopathy are the duration of chloroquine treatment and a daily dose of chloroquine >2.3 mg/kg of the patient's actual body weight.
Monitoring is of particular importance in patients over 60 years of age and in those with renal impairment.
Screening recommendations
Every patient planning to take chloroquine for at least 12 months should undergo a baseline ophthalmic examination within 6 months of commencing chloroquine and then annually. A fundus examination alone is not sufficient screening; the examination should involve automated visual fields testing and spectral-domain optical coherence tomography [6].
Other side effects
Cutaneous side effects
Chloroquine can cause:
- Blue–grey pigmentation, although less commonly than with quinacrine
- Urticaria
- Exfoliative dermatitis
- Psoriasiform eruption or flare of psoriasis
- Alopecia
- Photosensitivity
- Erythema annulare centrifugum
- Transverse pigmented nail bands.
Gastrointestinal side effects
Chloroquine can cause nausea, vomiting and diarrhoea; these side effects are often transient or resolve with dose reduction. Symptoms can be minimised by taking the medication with food.
Haematological effects
Haemolytic anaemia and reduced blood cell counts occur very rarely at recommended doses of chloroquine but may occasionally occur in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Neuromuscular effects
Neuromyopathy (diseases of the nerves and muscle tissues), myalgia, and fatigue have been reported with chloroquine use. Care should therefore be taken in patients with myasthenia gravis and dermatomyositis.
Drug interactions with antimalarial medications
The following medications should be reviewed when used concomitantly with chloroquine:
- Digoxin
- Methotrexate
- D-penicillamine
- Ciclosporin
- Beta blockers
- Amiodarone
- Penicillin
- Cimetidine
- Ritonavir
- Cholestyramine
- Antacids
- Aminoglycosides
- Corticosteroids
- Neostigmine
- Physostigmine.
Smoking
Smoking has been reported to inhibit the cytochrome P450 enzyme system, decreasing the efficacy of chloroquine [7].
What monitoring is required with chloroquine?
- The patient's full blood count should be checked prior to treatment and monthly for the first 3 months, then every 4–6 months [8].
- The patient's renal function should be checked at baseline, then after 1 month, after 3 months, and then every 4–6 months to assess for any changes in risk for adverse ocular effects (more frequent surveillance is needed if laboratory values are abnormal or with high-risk patients) [8].
- A pregnancy test should be undertaken in women of childbearing age at baseline and the patient should understand the need for reliable use of contraception during therapy.
- Consider testing for G6PD deficiency, especially in individuals originating from Africa, Asia, Oceania, or from Southern Europe.
- Every patient should undergo a baseline ophthalmic examination within the first 6 months of commencing chloroquine if the treatment is anticipated to be long-term. In the absence of specific risk factors, annual screening should then be performed.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Rodriguez-Caruncho C, Marsol IB. Antimalarials in dermatology: mechanism of action, indications, and side effects. Actas Dermosifiliogr 2014; 105: 243–52. DOI: 10.1016/j.adengl.2012.10.021. PubMed
- Mittal L, Zhang L, Feng R, Werth VP. Antimalarial drug toxicities in patients with cutaneous lupus and dermatomyositis: A retrospective cohort study. J Am Acad Dermatol 2018; 78: 100–6. DOI: 10.1016/j.jaad.2017.09.061. PubMed
- Ochsendorf FR. Use of antimalarials in dermatology. J Dtsch Dermatol Ges 2010; 8: 829–45. DOI: 10.1111/j.1610-0387.2010.07490.x. PubMed
- Marmor MF. Modern management of antimalarial usage and retinopathy. J Curr Ophthalmol 2017; 29: 143–4. doi: 10.1016/j.joco.2017.07.001. PubMed Central
- Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology 2016; 123: 1386–94. DOI: 10.1016/j.ophtha.2016.01.058. PubMed
- Yusuf IH, Lotery AJ, Ardern-Jones MR. Joint recommendations for retinal screening in long-term users of hydroxychloroquine and chloroquine in the United Kingdom, 2018. Br J Dermatol 2018; 174: 995–6. DOI: 10.1111/bjd.16782. PubMed
- Fernandez AP. Updated recommendations on the use of hydroxychloroquine in dermatologic practice. J Am Acad Dermatol. 2017; 76: 1176–82. DOI: 10.1016/j.jaad.2017.01.012. PubMed
- Wolverton SE. Comprehensive dermatologic drug therapy, 3rd edn. Philadelphia: Elsevier Saunders, 2012.
On DermNet
- Hydroxychloroquine
- Quinacrine
- Antimalarial medications in dermatology
- Oral medications for skin diseases
Other websites
- Recommendations on screening for chloroquine and hydroxychloroquine retinopathy — 2016 — American Academy of Ophthalmology
- Medicines data sheets – Medsafe New Zealand
- Special Access Scheme — Australian Therapeutic Goods Administration
