Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Mycophenolate mofetil — extra information
Mycophenolate mofetil
Authors: Vanessa Ngan, Staff Writer, 2003. Updated: Dr Kelvin Truong, Dermatology Research Fellow, Westmead Hospital, Sydney, Australia. Copy edited by Gus Mitchell. October 2021
Introduction Demographics Contraindications and precautions More information Benefits Disadvantages Side effects and risks
What is mycophenolate mofetil?
Mycophenolate mofetil is the prodrug form of mycophenolic acid, a potent immunosuppressive drug. Enteric-coated mycophenolate sodium is another form of mycophenolic acid used in clinical practice.
Who uses mycophenolate mofetil?
Mycophenolate mofetil is an immunosuppressant approved for to prevent rejection of solid organ (kidney, liver, heart) transplants.
Off-label use of mycophenolate mofetil is widespread in dermatology.
- Good evidence for a beneficial effect
- Immunobullous disorders such as bullous pemphigoid and pemphigus vulgaris
- Weak evidence and/or inconsistent response
- Atopic dermatitis, psoriasis
- Connective tissue diseases
- Vitiligo, small vessel vasculitis, lichen planus and its variants, erythema multiforme, pyoderma gangrenosum, granulomatosis with polyangiitis, acute febrile neutrophilic dermatosis, and many other inflammatory dermatoses.
Immunobullous diseases that may be treated with mycophenolate mofetil
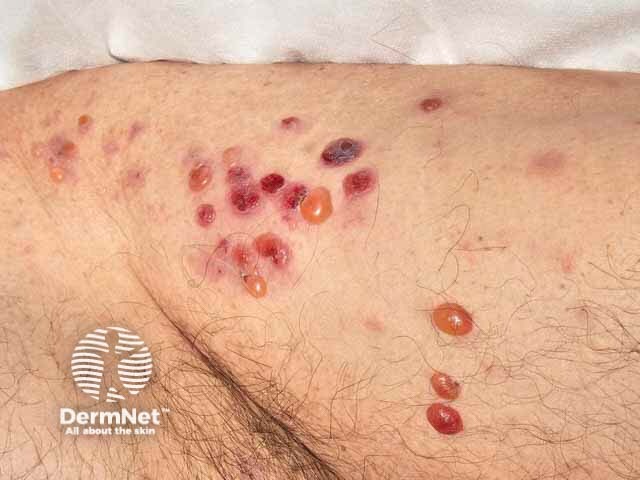
Bullous pemphigoid
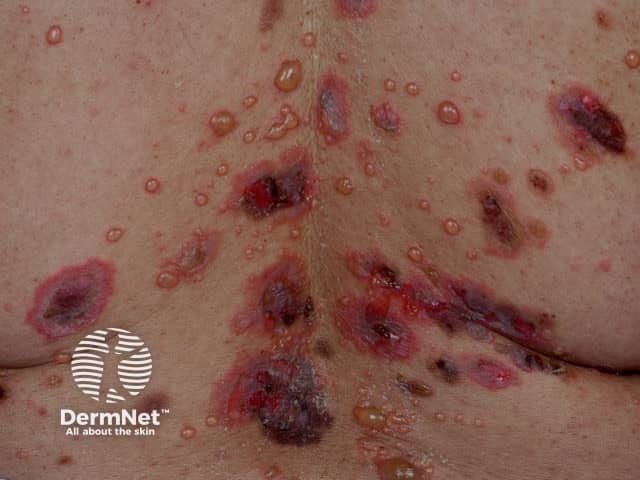
Pemphigus vulgaris
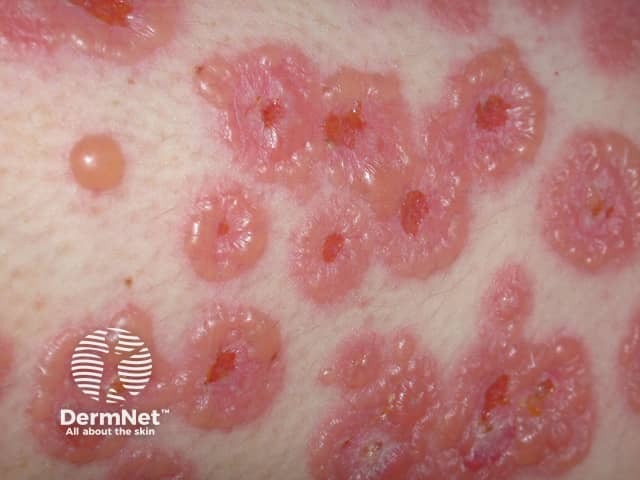
Linear IgA bullous disease
What are the contraindications and precautions with mycophenolate mofetil?
Absolute contraindications
- Allergy to the drug or its excipients
- Pregnancy
- Reported to cause spontaneous abortion, craniofacial malformations, cardiac malformations
- Women must use effective contraception starting four weeks before commencement, throughout treatment, and for six weeks after the last dose
- Men should use condoms during sexual intercourse throughout treatment and for 90 days after the last dose. Female partners of males treated with mycophenolate mofetil should also use effective contraception during their partner’s treatment and for 90 days after the partner’s last treatment dose.
Relative contraindications
- Lactation
- Peptic ulcer disease
- Liver disease
- Cardiopulmonary disease
Precautions
- Live vaccines should be avoided [see Immunisation in immunocompromised dermatology patients]
Tell me more about mycophenolate mofetil
Mechanism of action
- Reversible, selective, non-competitive inhibitor of the enzyme inosine monophosphate dehydrogenase (IMPDH)
- IMPDH is an important enzyme in the synthesis of purines
- Purine synthesis is essential for lymphocyte development and proliferation
- Blocking IMPDH inhibits T- and B-lymphocyte function
- Reduces lymphocyte proliferation, antibody formation, and cell-mediated immune response
Drug forms and dosing
Mycophenolate mofetil is available in oral and intravenous formulations.
A typical starting dose used in dermatology is 250 mg twice daily. If no improvement is observed after one month of therapy, the dose may be increased by 500 milligram increments up to a maximum of 3 g per day.
Dose reduction is required with chronic renal insufficiency as mycophenolic acid is mostly excreted via the kidneys.
Mycophenolate mofetil is often used in combination with other immunosuppressive drugs [see Drug-induced immunosuppression]
What are the benefits of mycophenolate mofetil?
- Well-tolerated
- Oral administration available
- Wide therapeutic range
What are the disadvantages of mycophenolate mofetil?
- Clinical response is slow
- Regular blood test monitoring is required
- Contraception is necessary for both males and females taking mycophenolate mofetil
- Drug interactions
- Reduced mycophenolate blood level
- Iron and calcium supplements, antacids, proton pump inhibitors
- Antibiotics including penicillins, cephalosporins, sulphonamides, macrolides, metronidazole
- Cholestyramine
- Increased mycophenolate blood level
- Probenecid
- Salicylates, phenytoin
- Mycophenolate may increase the blood level of other drugs
- Antivirals eg, aciclovir
- Reduced mycophenolate blood level
What are the side effects and risks of mycophenolate mofetil?
Side effects
Mucocutaneous side effects
- Mucocutaneous candidiasis is common
- Other skin infections — herpes varicella-zoster, atypical mycobacterial infection, tinea
- Urticaria
- Hand dermatitis
- Alopecia
-
Cutaneous squamous cell carcinoma — with long-term use of combination immunosuppressants such as post-transplant
- Recommend sun protection with SPF 50+ sunscreen and UPF 50+ clothing.
Mycophenolate mofetil can increase the risk of these skin infections
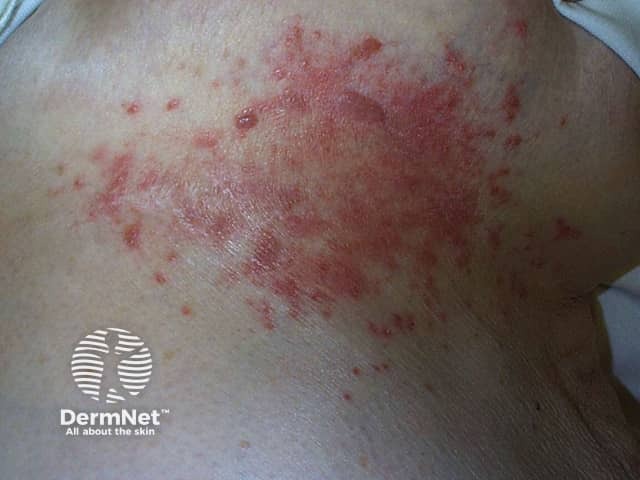
Candida intertrigo
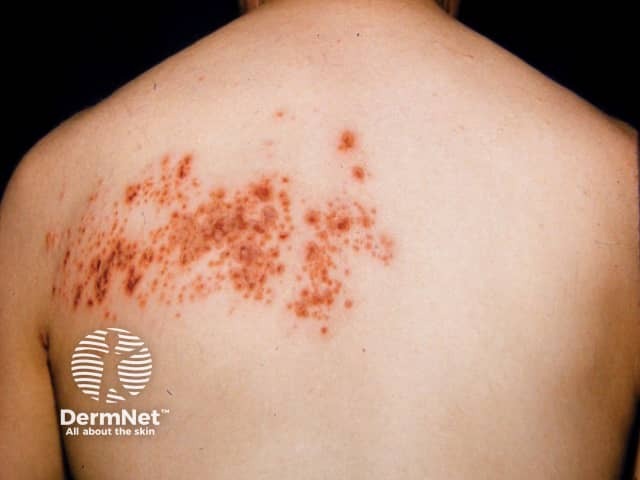
Herpes zoster
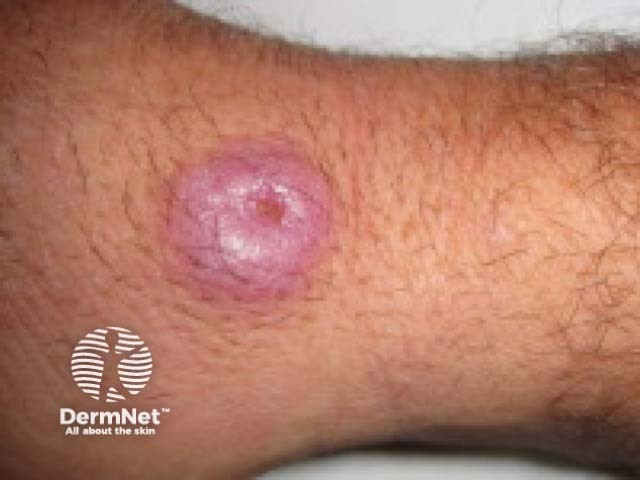
Atypical mycobacterial infection
Common side effects
- Nausea and vomiting, diarrhoea or constipation, abdominal cramps.
- Infections
- Bacterial — urinary tract infection, pneumonia
- Viral — cytomegalovirus (CMV), BK virus.
Uncommon but potentially serious side effects
- Opportunistic infections
- Bone marrow suppression — dose-related; reported in 5–9%; usually mild and reversible
- Epstein-Barr virus-associated lymphoproliferative disorders with long-term use in combination with other immunosuppressants such as post-transplant.
Monitoring during treatment
Before starting treatment
- Test for latent tuberculosis, hepatitis B and C
- Check immunity status eg, measles and varicella
- Baseline blood tests
During treatment
- Regular full blood count – weekly for the first month then every two weeks for two months, and monthly for the rest of the first year
- Liver and kidney function tests — every 2–4 weeks until dose is stabilised and then every 2–3 months
- Pregnancy test if appropriate in the first week
- Regular skin checks — patient should practice self skin examination
Discontinuation of treatment
Mycophenolate mofetil should be ceased if pregnancy occurs or:
- Lymphocyte count < 1.0x109/L
- Anaemia
- Thrombocytopenia
Bone marrow suppression, if mild, usually recovers with dose reduction or cessation of the mycophenolate mofetil.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
Bibliography
- Kitchin JE, Pomeranz MK, Pak G, Washenik K, Shupack JL. Rediscovering mycophenolic acid: a review of its mechanism, side effects, and potential uses. J Am Acad Dermatol. 1997;37(3 Pt 1):445–9. doi:10.1016/s0190-9622(97)70147-6. PubMed
- Mydlarski PR. Mycophenolate mofetil: a dermatologic perspective. Skin Therapy Lett. 2005;10(3):1–6. Journal
- Orvis AK, Wesson SK, Breza TS Jr, Church AA, Mitchell CL, Watkins SW. Mycophenolate mofetil in dermatology. J Am Acad Dermatol. 2009;60(2):183–202. doi:10.1016/j.jaad.2008.08.049. PubMed
- Park H. The emergence of mycophenolate mofetil in dermatology: from its roots in the world of organ transplantation to its versatile role in the dermatology treatment room. J Clin Aesthet Dermatol. 2011;4(1):18–27. PubMed Central
- Phan K, Smith SD. Mycophenolate mofetil and atopic dermatitis: systematic review and meta-analysis. J Dermatolog Treat. 2020;31(8):810–14. doi:10.1080/09546634.2019.1642996. PubMed
- Sharma P, Guragain A, Adhikari S, Dahal S, Dhungana B. Acute mycophenolate mofetil overdose managed conservatively. Cureus. 2021;13(8):e17417. doi:10.7759/cureus.17417. PubMed Central
- Strathie Page SJ, Tait CP. Mycophenolic acid in dermatology a century after its discovery. Australas J Dermatol. 2015;56(1):77–83. doi:10.1111/ajd.12259. PubMed
- Yamauchi PS, Rizk D, Kormeili T, Patnaik R, Lowe NJ. Current systemic therapies for psoriasis: where are we now?. J Am Acad Dermatol. 2003;49(2 Suppl):S66–S77. doi:10.1016/mjd.2003.550. PubMed
On DermNet
- Blistering skin conditions
- Drug-induced immunosuppression
- Immunisation in immunosuppressed dermatology patients
- Opportunistic infections
Other websites
- Mycophenolate — MedlinePlus
- Mycophenolate mofetil — British Association of Dermatologists
