Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Isotretinoin — extra information
Treatments Follicular disorder
Isotretinoin
Author: Hon A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand, 1999. Updated by Dr Amanda Oakley; Hon A/Prof Marius Rademaker, February 2016. Minor amendment by Ian Coulson, Dermatologist. July 2024.
Introduction
Uses
Contraindications
How to use
How it works
Dosage
Course duration
Drug interactions
General side effects and risks
Cutaneous side effects
Managing side effects
Dose-related side effects
Rare side effects
Monitoring
Contraption considerations for women
Isotretinoin and pregnancy
Contraption considerations for men
Efficacy
Recurrent treatment
Precautions for pilots
What is isotretinoin?
Isotretinoin (13-cis retinoic acid) is a vitamin-A derivative (retinoid). The liver naturally makes small quantities of isotretinoin from vitamin-A, but the prescribed drug is made synthetically.
Isotretinoin was developed in the 1950s, but only started being used in the mid 1970s. The original brand names were Accutane® and Roaccutane®, but there are now many generic versions on the market, of varying potency. In New Zealand, oral isotretinoin is available as 5 mg, 10 mg and 20 mg capsules (Oratane® brand). It is funded by PHARMAC on Special Authority application.
What is isotretinoin used for?
Isotretinoin is a very effective medication for the treatment of acne. Originally licensed for use in severe disease, it is increasingly prescribed for all grades of acne.
Isotretinoin is also useful for other follicular conditions, such as:
It is also prescribed off-label for many other skin diseases. Examples include:
- Discoid lupus erythematosus
- Granuloma annulare
- Grover disease
- Sarcoidosis
- Extensive actinic keratoses
- Prevention of cutaneous squamous cell carcinoma.
It has also been used as an adjuvant in neuroblastoma.
Contraindications to isotretinoin
Isotretinoin must not be taken in pregnancy, or if there is a significant risk of pregnancy.
Blood donation by males and females on isotretinoin is not allowed in case the blood is used for a pregnant woman.
There has been controversy regarding the use of isotretinoin in peanut allergic individuals - isotretinoin contains purified soya oil (where there is a possible risk of cross sensitivity) and in a study of 60 peanut sensitive individuals, none had a reaction to oral isotretinoin exposure.
Precautions when taking isotretinoin
- Isotretinoin should be used with caution during breastfeeding.
- Commercial pilots may be subject to flying restrictions if they take isotretinoin.
- High dose isotretinoin in very young children has been associated with premature epiphyseal closure, leading to shorter stature (this is not seen in the low dose used for the treatment of acne).
How does isotretinoin work?
In acne, isotretinoin:
- Reduces sebum production
- Shrinks the sebaceous glands
- Reduces follicular occlusion
- Inhibits the growth of bacteria
- Has anti-inflammatory properties.
What is the usual dose of isotretinoin?
The range of doses used each day for acne is less than 0.1 to over 1 mg/kg body weight. Some patients may only need a small dose once or twice a week. A course of treatment may be completed in a few months or continue for several years. For acne, some prescribers have targeted a total cumulative dose of 120–140 mg/kg, in the hope of reducing relapse, but the evidence for this remains controversial. The general trend has been to use lower dosages, unrelated to body weight (eg, 10 mg/day).
The individual dose prescribed by the dermatologist depends on:
- Prescriber preference
- Patient body weight
- The specific condition being treated
- The severity of the skin condition
- Response to treatment
- Other treatment used at the same time
- Side effects experienced.
Isotretinoin is better taken with water or milk after food to help with its absorption. It may be taken on an empty stomach, but absorption may be halved. There is no particular advantage in splitting the dose over the day. A newer formulation (isotretinoin-lidose) can be taken without food.
For how long is isotretinoin taken?
Most patients should be treated until their skin condition clears and then for a further few months. However, courses have often been restricted to 16–30 weeks (4–7 months) to minimise the risk of teratogenicity (risk of congenital abnormalities), and to comply with local regulatory authorities. Isotretinoin may be prescribed for years, usually in low dose or intermittently.
Drug interactions with isotretinoin
Care should be taken with the following medications:
- Vitamin-A (retinoic acid): side effects are cumulative and could be severe. Beta-carotene (provitamin-A) is permitted.
- Tetracyclines (including doxycycline, minocycline): these could increase the risk of headaches and blurred vision due to raised intracranial pressure.
- Warfarin: monitor INR carefully.
What are the side effects and risks of isotretinoin?
The side effects of isotretinoin are dose dependent; at 1 mg/kg/day, nearly all patients will have some side effects, whereas, at 0.1 mg/kg/day, most patients will not. The range and severity of the side effects also depend on personal factors and the disease being treated.
Patients with significant liver or kidney disease, high blood fats, diabetes and depression may be advised not to take isotretinoin or to be on a lower dose than usual and to have regular follow-up visits.
Cutaneous and mucocutaneous side effects
Most of the side effects due to isotretinoin are cutaneous or mucocutaneous and relate to the mode of action of the drug. When side effects are troublesome, isotretinoin may need to be withheld or the dose reduced. The most common side effects are listed here:
- Acne flare-up (particularly if starting dose is > 0.5 mg/kg/day)
- Dry lips, cheilitis (sore, cracked or scaly lips) (100% of patients on 1 mg/kg/day)
- Dry skin, fragile skin, eczema/dermatitis (itchy, red patches of skin)
- Note: atopic eczema may improve.
- Increased sweating
- Dry nostrils, epistaxis (nose bleeds)
- Dry, watery or irritable eyes (especially in contact lens wearers), conjunctivitis, keratitis
- Dry anal mucosa, bleeding at the time of a bowel motion
- Dry genitals, dyspareunia (discomfort during intercourse)
- Facial erythema
- Sunburn on exposure to the sun
- Temporary hair loss
- Brittle nails
- Pyogenic granuloma
- Skin infections: impetigo, acute paronychia.
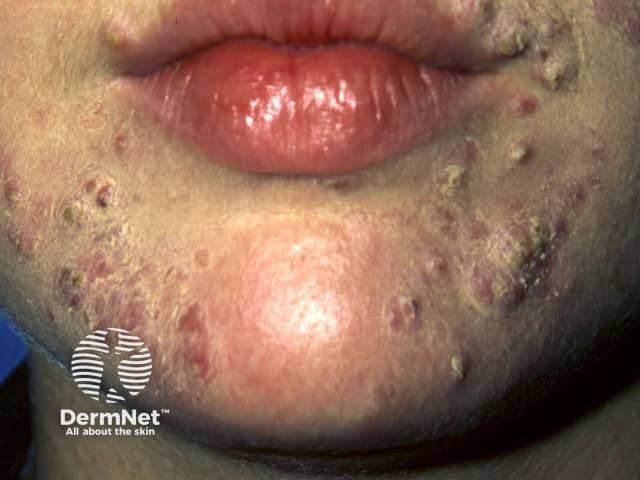
Acne flare
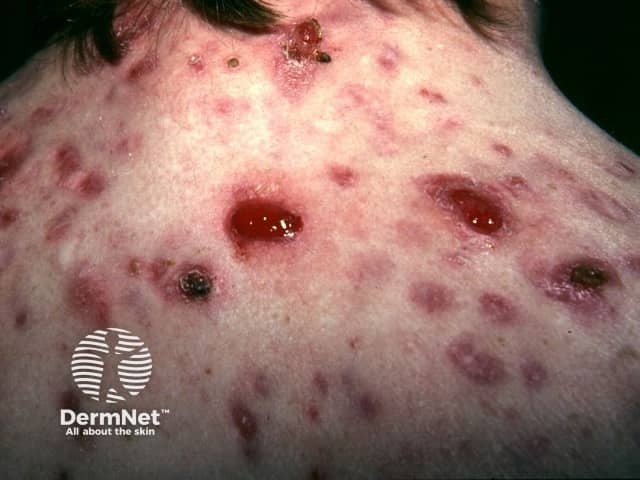
Granulomas
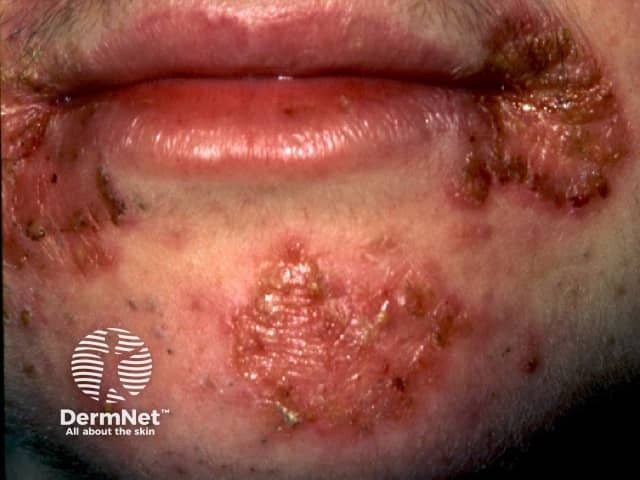
Impetigo
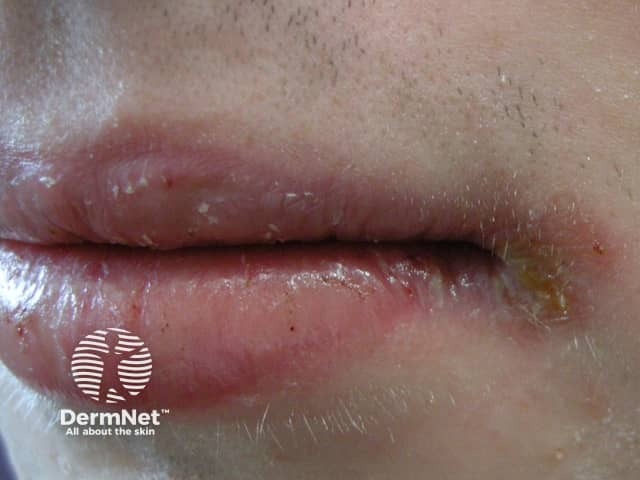
Cheilitis
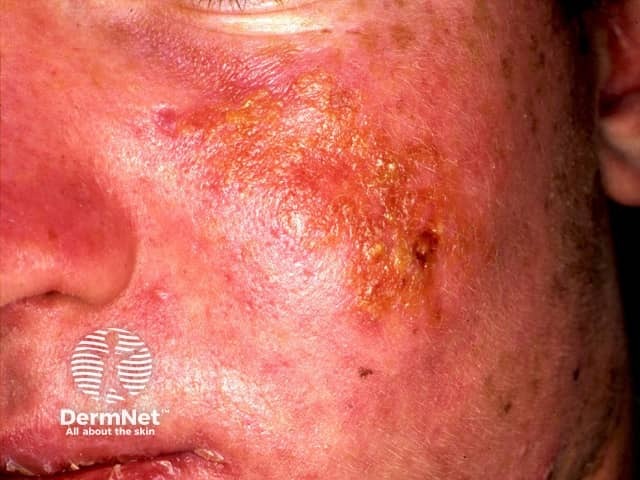
Sunburn
Treatment of mucocutaneous side effects
- Reduce the dosage (eg, to 5–10 mg/day).
- Emollients, lip balm, petroleum jelly, sunscreen, eye drops and lubricants should be applied frequently and liberally when needed.
- Dermatitis can be treated with topical steroids.
- Take short, cool showers without using soap.
- Use mild or diluted shampoo.
- Do not start wearing contact lenses for the first time.
- Do not have elective eye surgery while on isotretinoin or for 6 months afterwards.
- Do not have mechanical dermabrasion or ablative laser treatments (eg, CO2 resurfacing) while on isotretinoin or for 6 months afterwards. Other laser and light treatments may be performed with care.
- Shave rather than wax.
- Topical and/or oral antibiotics may be prescribed for impetigo.
Other dose-related side effects of isotretinoin
- Headache.
- Myalgia (muscle aches) and arthralgia (joint aches), especially after exercise.
- Tiredness (lethargy and drowsiness).
- Disturbed night vision and slow adaptation to the dark. Drivers may experience increased glare from car headlights at night.
- Hypertriglyceridaemia (high levels of triglyceride in the blood), usually of no clinical relevance.
- Irregular or heavy menstrual periods.
Rare side effects of isotretinoin
The causality of the listed side effects may not have been confirmed.
- Severe headache with blurred vision due to raised intracranial pressure.
- Mood changes and depression. Note: depression is more often related to the skin condition being treated or other health or psychosocial problems. Antidepressant medications may be helpful. See Isotretinoin and mood below.
- Sexual dysfunction (eg, erectile dysfunction and decreased libido).
- Corneal opacities and cataracts.
- High-tone deafness.
- Accelerated diffuse interstitial skeletal hyperostosis (bony change).
- Abnormal liver function tests or symptomatic hepatitis.
- Diarrhoea or bleeding from the bowel. A large review suggested that use of isotretinoin for over a year may increase the risk inflammatory bowel disease development.
- Pancreatitis.
- Allergy to isotretinoin causing liver disease and a febrile illness.
Treatment of systemic side effects
- Drink minimal alcohol.
- Take paracetamol for headache and for mild aches and pains.
- Seek medical attention early, if unwell.
Isotretinoin and mood
The effect of isotretinoin on mood is a very controversial area; the majority of acne sufferers have improved mood commensurate with the improvement in their acne. Rarely, isotretinoin induces depression and low mood, when on stopping the drug, depression lifts but returns with reintroduction of the medication. Acne itself can be the source of distress, anxiety and depression. In rare instances, suicidal ideation and completed suicide occurs during isotretinoin treatment, but this can also rarely be the case in people taking antibiotics for acne, so it is difficult to be certain whether the changes are due to the disease, scarring, the drug, or something else.
The largest meta-analysis reviewing the data of 1.6 million people identified the 1 year absolute risk of suicide, suicidal ideation, completed suicide, and self harm to be less than 0.5% each, and depression 3.8%, all being lower than the risk in the general adolescent population, suggesting that there was no increased relative risk of psychiatric condition in isotretinoin users. In the 2-4 years after isotretinoin the psychiatric illness rate was lower than those who had not had isotretinoin. There was a slightly increased risk of depression in those who had isotretinoin and had a past history of depression. Patients and their families should however remain aware of rare psychiatric events and monitored by their doctors for them.
Monitoring isotretinoin
Pregnancy must be excluded before and during treatment with isotretinoin.
In an otherwise healthy individual, blood tests are generally not needed, but monitoring may be a variable prescribing requirement in some countries. If using high dose isotretinoin (1 mg/kg/day), a prolonged course (> 12 months), or if a patient has specific risk factors (eg, family history of dyslipidaemia, viral hepatitis, or high alcohol intake), these tests are indicated prior to treatment and at intervals:
- Cholesterol and triglyceride levels
- Liver function tests
- Blood count.
Contraception in females considering isotretinoin
Isotretinoin must not be taken in pregnancy because of a very high risk of serious congenital abnormalities in the baby. Caution needs to be used during breastfeeding as it enters the breast milk and might affect the baby.
All females who could biologically have a child should take the following precautions during treatment with isotretinoin and for four weeks after the medication has been discontinued:
- Abstinence. The most reliable method of avoiding pregnancy is not to have sex. No method of contraception is completely reliable. "Natural" family planning is particularly risky.
- If sexually active, two reliable methods of contraception should be used. Discuss contraception with your doctor (general practitioner, family planning specialist, gynaecologist or dermatologist). The combined oral contraceptive, IUD (intrauterine device), a progesterone implant, or medroxyprogesterone injection may be suitable.
- The low-dose progesterone mini-pill on its own is not recommended.
A prescription for emergency contraception may be available from a medical practitioner (GP or family planning clinic) or accredited pharmacy. It prevents 85% of pregnancies if taken within 72 hours of unprotected sexual intercourse.
If contraception fails, termination of pregnancy (an abortion) may be advised if pregnancy arises during treatment with isotretinoin or within a month of discontinuing it.
What happens if a pregnant woman takes isotretinoin?
Isotretinoin has a very high chance of resulting in a spontaneous miscarriage or a severe birth deformity if a fetus is exposed to it during the first half of pregnancy. The deformities affect the growth of tissues developing at the time of exposure to the drug:
- Cranium (skull and brain)
- Cardiac (heart)
- Eye, ear
- Limbs.
No contraceptive precautions are necessary for men
Isotretinoin has no effect on sperm or male fertility and has not been shown to cause birth defects in children fathered by men taking it.
Does acne ever fail to clear on isotretinoin?
Although isotretinoin is usually very effective for acne, occasionally it responds unexpectedly slowly and incompletely. Poor response is associated with:
- Macrocomedones (large whiteheads)
- Nodules (large, deep inflammatory lesions)
- Secondary infection
- Smoking
- Polycystic ovarian syndrome
- Younger age (< 14 years).
Options available to slow responders include:
- Electrocautery of comedones
- A prolonged course of isotretinoin
- Additional treatment with oral antibiotics and oral steroids.
Can isotretinoin be used again if acne recurs?
At least fifty percent of patients with acne have a long-lasting response after a single adequate course of isotretinoin. In others, acne may recur a few months to a few years after the medication has been discontinued. Relapse is more common in females than in males, and in patients > 25 years of age. These patients may receive further courses of isotretinoin.
Long-term treatment (> 1 year) is often used for patients with:
- Persistent acne
- Seborrhoea
- Rosacea
- Scalp folliculitis
- Skin cancer.
Special precautions for pilots considering isotretinoin
Good night vision is important for airline pilots and those flying after dark. Night vision may be affected by isotretinoin. Pilots taking isotretinoin or considering a course of isotretinoin must report to their national aviation authority to discuss how this treatment affects their flying privileges.
In New Zealand, this is the Civil Aviation Authority of New Zealand. Civil aviation licence holders, including Air Traffic Controllers, have an obligation under section 27 C of the Civil Aviation Act to ground self and report to CAA in case of any change in medical condition that may affect flight safety. CAA considers the use of isotretinoin to be a change in medical condition.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
Bibliography
- Advice on the safe introduction and continued use of isotretinoin in acne in the UK 2010 – MJD Goodfield, NH Cox, A Bowser, JC McMillan, LG Millard, NB Simpson, AD Ormerod, BJD, Vol. 162, No. 5, June 2010 (p1172–9)
- Mollan SP, Woodcock M, Siddiqi R, Huntbach J, Good P, Scott RA. Does use of isotretinoin rule out a career in flying? Br J Ophthalmol. 2006 Aug;90(8):957–9. PubMed.
- Ruth E, Heraghty F, Flynn N, et al. No evidence of isotretinoin sensitization in peanut-allergic children: a cross-sectional study. Br J Dermatol. 2023;189(4):481-482. PubMed
- Tan NKW, Tang A, MacAlevey NCYL, Tan BKJ, Oon HH. Risk of Suicide and Psychiatric Disorders Among Isotretinoin Users: A Meta-Analysis [published correction appears in JAMA Dermatol. 2024 Jan 1;160(1):118]. JAMA Dermatol. 2024;160(1):54-62. PubMed
- Ahmed A, Liaquat A, Raza S, et al. Association of inflammatory bowel disease incidence with isotretinoin usage: A meta-analysis and systematic review. J Am Acad Dermatol. 2024;91(5):949-951. PubMed
On DermNet
Other websites
- Consumer medicine information and data sheets — Medsafe
- Drugs, Herbs and Supplements — MedlinePlus
- Isotretinoin — British Association of Dermatologists
- Isotretinoin: Patient Handouts — The Society for Pediatric Dermatology
- Isotretinoin — SafeRx
- Isotretinoin — American Academy of Dermatology
Books about skin diseases
