Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Tacrolimus — extra information
Tacrolimus
Authors: Vanessa Ngan, Staff Writer, 2004. Updated: Dr Kelvin Truong, Dermatology Research Fellow, Westmead Hospital, Sydney, Australia; Dr Martin Keefe, Dermatologist, Christchurch, New Zealand. Copy edited by Gus Mitchell. September 2021.
Introduction
Uses
Contraindications
More information
Benefits
Disadvantages
Side effects and risks
What is tacrolimus?
Tacrolimus is a macrolide calcineurin inhibitor immunosuppressant drug available as a topical ointment, oral capsule, and intravenous injection. It was initially isolated from the soil fungus Streptomyces tsukabaenis.
Who uses tacrolimus?
Systemic tacrolimus
- Solid organ transplant — kidney, heart, lung – approved for adults and children to prevent post-transplant organ rejection
- Off-label use in dermatology – graft versus host disease, hidradenitis suppurativa, paediatric systemic lupus erythematosus
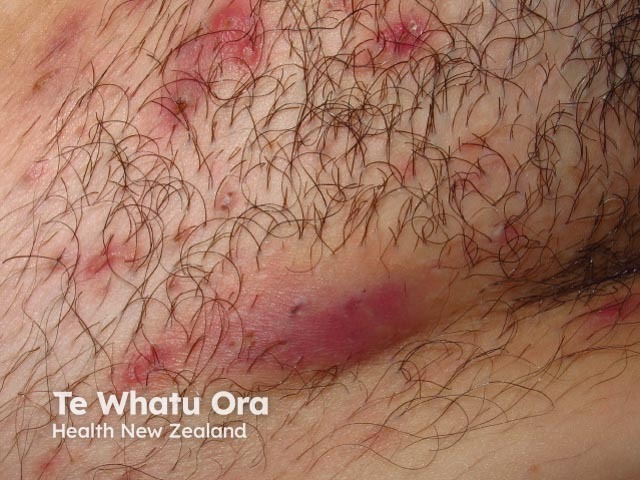
Hidradenitis suppurativa
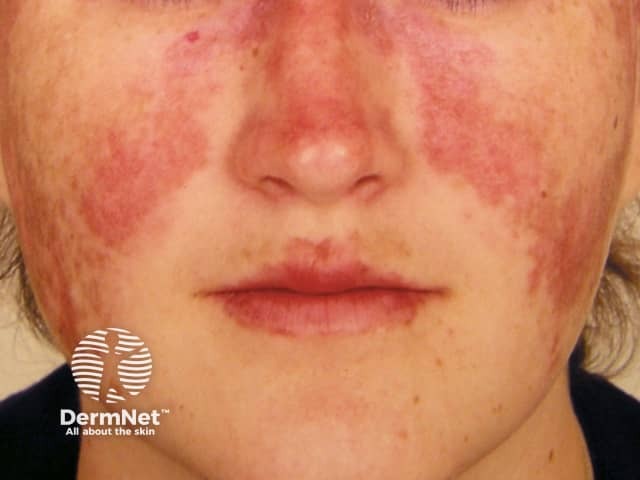
Systemic lupus erythematosus
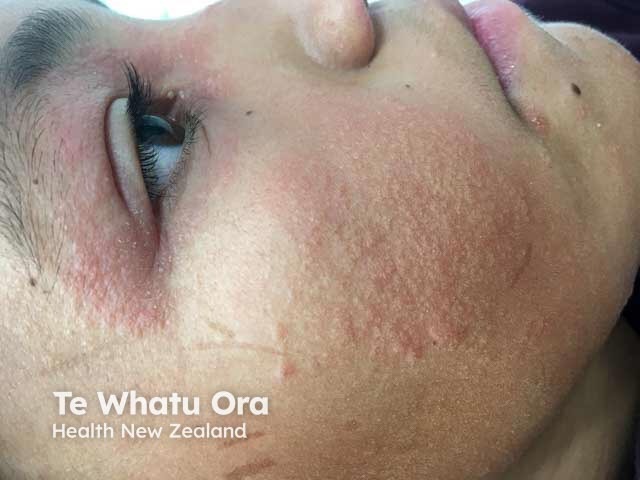
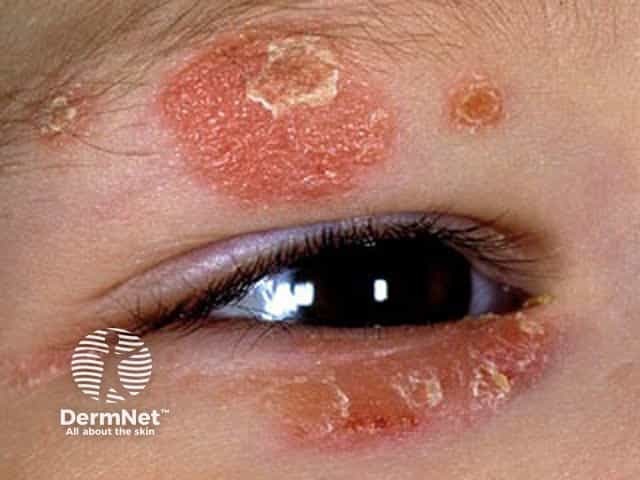
Face and eyelid atopic dermatitis in a child
Topical tacrolimus
- Atopic dermatitis — adults and children over the age of 2 years
- Off-label uses in dermatology include:
- Double-blind clinical trials
- Seborrhoeic dermatitis, oral lichen planus (as a mouth rinse), pruritus ani, facial psoriasis, flexural psoriasis
- Clinical trials
- Small trials of at least 20 people
- Double-blind clinical trials
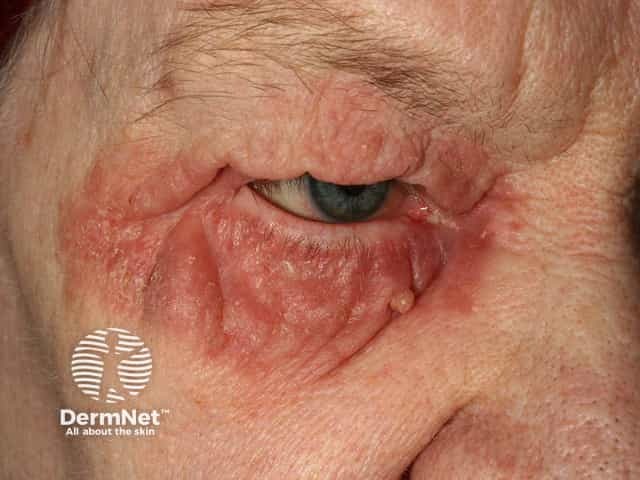
Seborrhoeic dermatitis
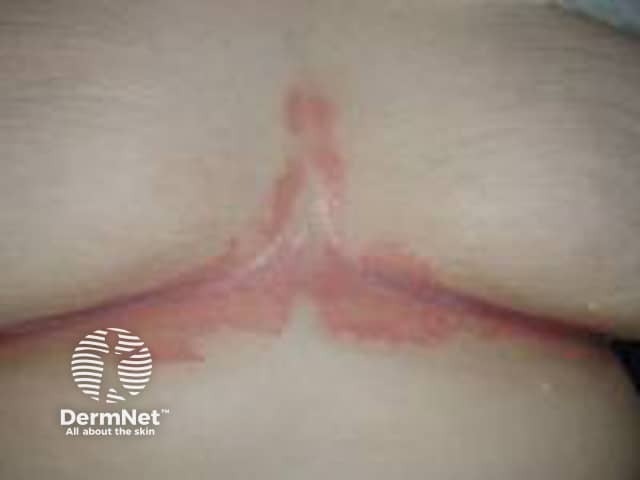
Flexural psoriasis
Facial psoriasis
What are the contraindications with tacrolimus?
Contraindications to systemic and topical tacrolimus
- Hypersensitivity to tacrolimus and other macrolides such as erythromycin, or product excipients
- Pregnancy and lactation – Pregnancy Category C
- Concurrent use of ciclosporin
Additional contraindications to topical tacrolimus
- Children under the age of 2 years
- Immunocompromised adults and children
- Active bacterial skin infection
- Application to malignant or premalignant skin lesions
- Netherton syndrome due to significant percutaneous absorption
Tell me more about tacrolimus
Tacrolimus is a lipophilic molecule; for topical use it is formulated as an ointment. Percutaneous absorption is minimal except where there is an epidermal barrier defect such as in active atopic dermatitis. As disease activity settles, absorption through the skin reduces.
Tacrolimus is metabolised by cytochrome P450 in the liver. Tacrolimus is not metabolised in the skin.
Polymorphisms in the CYP3A5 gene affect the bioavailability of systemic tacrolimus with CYP3A5 non-expressors requiring a higher dose of systemic tacrolimus post-transplant.
Mechanism of action of tacrolimus
Tacrolimus suppresses the cell-mediated immune response.
- Tacrolimus binds to FKBP-12 (an intracellular protein) preventing activation of calcineurin phosphatase, thus inhibiting dephosphorylation of nuclear factor of activated T-cells (NFAT) and suppressing activity of genes that code for IL-2.
- Tacrolimus also inhibits transcription of genes which encode IL-3, IL-4, IL-8, GM-CSF, and TNF-α, all of which are involved in the early stages of T-cell activation.
- Tacrolimus inhibits the release of preformed mediators from skin mast cells and basophils, and downregulates the expression of the IgE receptor FcεRI on Langerhans cells.
How to use tacrolimus
Systemic tacrolimus
- Following a solid organ transplant, tacrolimus is initially given as an intravenous continuous infusion until able to swallow.
- Oral tacrolimus is available as a twice daily immediate-release, a once-daily extended-release capsule, or granule.
Topical tacrolimus
- A thin layer of tacrolimus ointment is applied to the affected areas twice each day. It can be used on any affected skin site, including the face and folds, but mucous membranes should be avoided.
- Topical steroids may be better for an acute flare with topical tacrolimus introduced as the dermatitis improves.
- Emollients are usually prescribed concurrently, with tacrolimus applied at least two hours later.
- Topical tacrolimus can be continued twice each week for maintenance.
What are the benefits of tacrolimus?
Systemic tacrolimus
- Less nephrotoxic than ciclosporin
- Less frequent gingival enlargement and hypertrichosis than ciclosporin
Topical tacrolimus
- Efficacy is equivalent to moderate-to-potent topical steroids
- Does not cause skin atrophy so can be used on the face and skin folds
- No association with glaucoma or cataracts
- Response is usually seen within one week
What are the disadvantages of tacrolimus?
Systemic tacrolimus
- Narrow therapeutic range
- Risk of toxicity and requirement for monitoring of serum levels
- May unmask latent Lynch syndrome
- Drug interactions include posaconazole and erythromycin — tacrolimus dose must be reduced if these are prescribed to avoid toxicity
- Atypical haemolytic uraemic syndrome
Topical tacrolimus
- Enhanced absorption across mucous membranes
- Tinea incognito if applied to a dermatophyte skin infection
- Steroid rosacea-like face rash with prolonged topical application
- Toxicity can, rarely, follow topical use
What are the side effects and risks of tacrolimus?
The Food and Drug Administration (FDA) has a black box warning for a possible increase in cancer risk including skin cancers and lymphoma. Evidence is conflicting with topical use so the risk, if any, is probably very small.
Systemic tacrolimus
- Mucocutaneous
- Itch (36%)
- Skin rashes (24%)
- Hyperhidrosis
- Alopecia and hypertrichosis
- Photosensitivity
- Hyperpigmentation
- Stevens/Johnson syndrome/toxic epidermal necrolysis, urticaria
- Other
- Common symptoms — diarrhoea, headache, tremor, nausea
- Symptoms of toxicity include nausea, paraesthesia, light-headedness
- Hypertension, hyperglycaemia, anaemia, renal impairment
Topical tacrolimus
- Mucocutaneous
- Application site irritation, stinging, burning, itch
- Contact dermatitis
- Folliculitis
- Focal hypertrichosis and trichomegaly at application site
- Reactivation of herpes simplex
- Increased risk of candidiasis with use in anogenital area
- Other
- Flu-like symptoms including headache and fever
- Application site redness following alcohol ingestion
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
Bibliography
- Ali FR, Lyon CC. Tacrolimus toxicity following topical treatment of perianal Crohn disease: an admonitory anecdote. J Crohns Colitis. 2013; 7(12): e713. Journal
- Cury Martins J, Martins C, Aoki V, Gois AF, Ishii HA, da Silva EM. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015;2015(7):CD009864. doi:10.1002/14651858.CD009864.pub2. Journal
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–70. doi:10.1016/j.jaad.2020.07.087. Journal
- Ghislain PD, De Decker I, Lachapelle JM. Efficacy and systemic absorption of topical tacrolimus used in pyoderma gangrenosum. Br J Dermatol. 2004;150(5):1052–3. doi:10.1111/j.1365-2133.2004.05914.x. PubMed
- Madan V, Griffiths CE. Systemic ciclosporin and tacrolimus in dermatology. Dermatol Ther. 2007;20(4):239–50. doi:10.1111/j.1529-8019.2007.00137.x. PubMed
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153–63. doi:10.2147/PTT.S101233. Journal
- Ponte GM, Baidal DA, Romanelli P, et al. Resolution of severe atopic dermatitis after tacrolimus withdrawal. Cell Transplant. 2007;16(1):23–30. doi:10.3727/000000007783464524. Journal
- Sun SL, Liu JJ, Zhong B, et al. Topical calcineurin inhibitors in the treatment of oral lichen planus: a systematic review and meta-analysis. Br J Dermatol. 2019;181(6):1166–76. doi:10.1111/bjd.17898. PubMed
- Taieb A, Alomar A, Böhm M, et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168(1):5–19. doi:10.1111/j.1365-2133.2012.11197.x. Journal
- Tavira B, Coto E, Díaz-Corte C, et al. Pharmacogenetics of tacrolimus after renal transplantation: analysis of polymorphisms in genes encoding 16 drug metabolizing enzymes [published correction appears in Clin Chem Lab Med. 2011 Jun;49(6):1087. Garciá, Eliecer Coto [corrected to Coto, Eliecer]; Alvarezca, Victoria [corrected to Alvarez, Victoria]]. Clin Chem Lab Med. 2011;49(5):825–33. doi:10.1515/CCLM.2011.143. PubMed
On DermNet
- Cutaneous adverse reactions to calcineurin inhibitors
- Drug-induced immunosuppression
- Pimecrolimus
- Topical calcineurin inhibitor
- Treatment of atopic dermatitis
Other websites
- Calcineurin inhibitors — British Association of Dermatologists
- Protopic Medication Guide — FDA
- Tacrolimus. Aust Prescr 1998;21:80–3.
- Tacrolimus — US Food & Drug Administration
