Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Neurocutaneous melanosis — extra information
Lesions (benign) Genetic Pigmentary disorders
Neurocutaneous melanosis
Last Reviewed: January, 2024
Authors: Dr Gabriela E. Beraja, DO, MS, OhioHealth Doctors Hospital, USA; Dr Michaele A. Garrison, DO, Department of Neurology, University of Florida, USA; Dr Jay Nguyen, DO, Kansas City University GME Consortium/ADCS; and Dr Jere J. Mammino, DO, FAAD, FAOCD, Dermatologist, Kansas City University GME Consortium/ADCS, USA; Dr Libby Whittaker, DermNet Medical Writer (2024).
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Demographics
Causes
Clinical features
Variation in skin types
Diagnosis
Differential diagnoses
Treatment
Prevention
Outcome
What is neurocutaneous melanosis?
Neurocutaneous melanosis (NCM) is a rare neurological disorder involving the abnormal accumulation of melanin within the central nervous system (CNS), accompanied by the presence of congenital melanocytic naevi (nevi).
NCM is thought to arise from the aberrant proliferation of neural crest-derived melanocytes. This leads to the development of benign or malignant melanocytic tumours within the CNS, along with at least one congenital melanocytic naevus (CMN).
Those with NCM may remain asymptomatic or show neurological signs and symptoms, most frequently seen in the first two years of life.
Neurocutaneous melanosis may also be referred to as neurocutaneous melanocytosis, neurocutaneous melanomatosis or neurocutaneous melanosis syndrome.
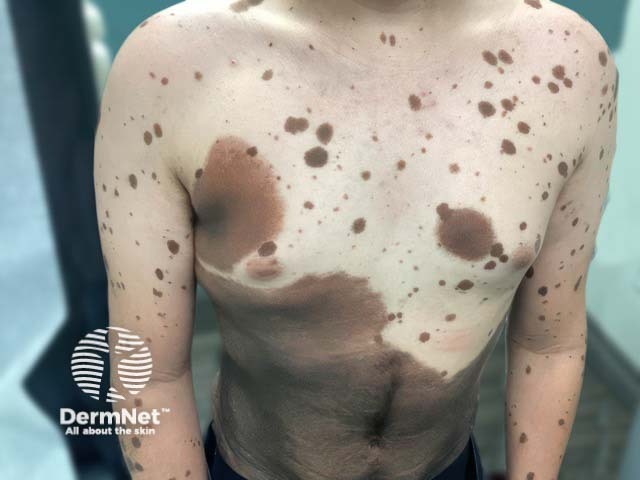
A giant melanocytic naevus and multiple satellite lesions in NCM (NCM-patient1)
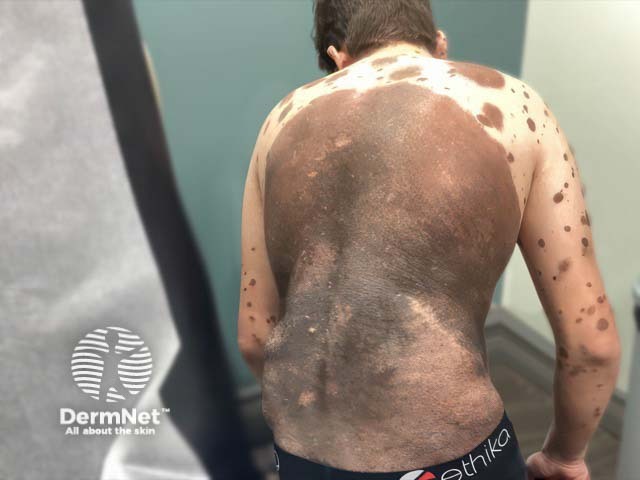
A giant melanocytic naevus and multiple satellite lesions in NCM (NCM-patient1)
Who gets neurocutaneous melanosis?
The prevalence of NCM is estimated at 1:50,000–1:200,000, with no clear gender predominance. Symptomatic NCM has been reported to manifest in approximately one-third to half of cases, typically in the first two years of life.
The estimated risk of NCM developing in individuals with large and giant congenital melanocytic naevus (CMN) is 10–33%.
Features of CMN that are associated with an increased risk of NCM include:
- Multiple CMN, including a greater number of smaller ‘satellite’ lesions
- Giant (>40 cm) naevus size
- Posterior axis location (head, neck, and paraspinal regions) — possibly a proxy for naevus size.
What causes neurocutaneous melanosis?
NCM is caused by an error within the neural crest cells of the ectoderm that results in the proliferation of mutated melanin-producing cells in the skin and leptomeninges.
In 2001, Takayama et al suggested this mutation within neural crest cells stemmed from atypical expression of hepatocyte growth factor/scatter factor.
Kinsler et al (2013) discovered another contributing factor to NCM, involving somatic mosaicism of a postzygotic mutation in codon 61 of NRAS, a developmental gene and oncogene playing a pivotal role in the regulation of crucial cell signalling pathways.
Building upon this research, Pawlikowski et al (2015) reasoned that activated Wnt signalling, in addition to the NRAS mutation, could contribute to the diverse phenotype observed in cases of NCM.
What are the clinical features of neurocutaneous melanosis?
Cutaneous features
NCM tends to occur in patients with multiple medium-sized (1.5–20 cm) or large (>20–40 cm), or at least one giant (>40 cm) congenital melanocytic naevi (CMN). Note in children, these categorisations are based on projected size of the CMN in adulthood. Earlier size classifications differed with ‘giant’ CMN being previously considered >20 cm in diameter.
Multiple smaller naevi, sometimes referred to as satellite lesions, increase the risk of developing NCM.
NCM is also more likely in those with a ‘bathing trunk’ CMN distribution in the truncal, sacral, and perineal areas.
CMN may have associated hypertrichosis and verrucous-like texture and can develop papules and nodules.
Non-cutaneous features
Symptoms of neural melanosis, if they occur, may include manifestations of increased intracranial pressure that usually transpire within the first two years of life, such as:
- Seizures
- Headaches
- Cranial nerve palsies
- Rapidly changing head circumference secondary to hydrocephalus
- Radiculopathy
- Symptoms of spinal cord compression
- Developmental delay.
Neurocutaneous melanosis can also cause stillbirth.
Dandy-Walker malformation (a rare congenital malformation of the cerebellum and fourth ventricle) has been observed in approximately 10% of individuals diagnosed with NCM. There also have been reports of NCM cases co-occurring with Chiari malformation, arachnoid cysts, choroid plexus papilloma, cerebellar astrocytoma, and spinal dysraphism, indicating a broader spectrum of associated conditions.
How do clinical features vary in differing types of skin?
No specific data published on this.
What are the complications of neurocutaneous melanosis?
Both skin and CNS lesions are at risk of malignant transformation, with leptomeningeal tumour transformation occurring in 40–60% of symptomatic cases. A giant CMN is associated with an estimated 3–8% lifetime risk of developing melanoma.
NCM can be further complicated by hydrocephalus, seizures, cranial nerve dysfunction, and the sequelae of spinal cord and root involvement.
How is neurocutaneous melanosis diagnosed?
In 1991, Kadonaga and Frieden revised the criteria for NCM to include:
- Large/giant (≥20 cm in adults; or in infants ≥6 cm on the trunk or ≥9 cm on the head) or multiple (≥3) congenital melanocytic naevi (CMN) in association with meningeal melanosis or melanoma
- No evidence of cutaneous melanoma except when examined meningeal lesions are histologically benign
- No evidence of meningeal melanoma except when cutaneous lesions are histologically benign.
Points 2 and 3 help to distinguish neurocutaneous melanosis from cases of metastatic melanoma eg, cutaneous melanoma that has metastasised to the CNS.
Magnetic resonance imaging (MRI)
The preferred imaging modality is an MRI brain and spine with the recommendation of prompt evaluation, ideally within the first 4–6 months of life, for patients with one or more of the following features:
- Giant CMN (>40 cm)
- Multiple medium or large CMN
- ≥10 ‘satellite’ lesions
- Any CMN + neurological signs or symptoms.
Solitary small, medium, and large CMN are generally considered low risk for NCM, therefore MRI brain and spine may not be indicated in these patients. There is debate around this and some suggest imaging all patients with large CMN (>20 cm) or >1 CMN.
MRI appears to be most sensitive to melanosis in the first few months of life and is the best test to evaluate the involvement of the leptomeninges. Non-contrast MRI may be attempted on young infants using a ‘feed and swaddle’ technique that avoids the need for general anaesthetic.
Melanocyte deposition appears hyperintense on T1-weighted MRI due to its paramagnetic properties, with focal shortening of T1 relaxation time compared with T2. Neural melanosis is most commonly seen in the amygdala, and sometimes the inferior cerebellum, ventral medulla, pons, cerebral peduncles, or the upper cervical spinal cord. Deep parenchymal structures are usually spared.
MRI findings for malignant CNS lesions may include a mass with nodular or thick plaque-like contrast enhancement, oedema, rapid growth, necrosis, or haemorrhage. However, MRI alone often cannot distinguish benign from malignant neural melanosis.
Other investigations
Cerebrospinal fluid (CSF) analysis and the use of ultrasound have been reported. Transfontanellar ultrasonography may reveal increased echogenicity of the amygdala if lesions are present and can aid in the detection and monitoring of hydrocephalus.
What is the differential diagnosis for neurocutaneous melanosis?
Depending on MRI brain and spine findings, differential diagnoses could potentially include:
- Other melanin-containing lesions, including metastatic melanoma from a cutaneous primary tumour
- Leptomeningeal carcinomatosis
- Non-melanocytic haemorrhagic tumours
- Urbach–Wiethe disease (lipoid proteinosis)
- Subarachnoid haemorrhage
- Meningitis.
What is the treatment for neurocutaneous melanosis?
General measures
Specialist dermatologist follow-up is important. All children with a confirmed diagnosis of neurocutaneous melanosis should also be referred to a paediatric neurologist. Neurosurgical input may also be needed.
It is recommended to conduct lifelong monitoring, including visual inspection +/- dermoscopy and palpation of each CMN every 6–12 months, and palpation for lymphadenopathy. Baseline and serial photographs and detailed mole-mapping can help track any alterations. Any palpable nodules or changes such as rapid growth or new induration or ulceration warrant consideration of histological examination. More frequent visits may be recommended during infancy and puberty, which are times of increased naevus change and growth.
It may be reasonable to contemplate surgical excision / removal of CMN in children ≥6 months of age. However, CMN removal does not fully protect against the onset of melanoma as half of these are derived from melanocytes elsewhere in the body (eg, CNS). Careful consideration of risks and benefits of CMN removal is needed, including factors such as the size and location of the naevus, patient and/or family preference, and the overall prognosis.
Specific measures
There is no definitive treatment for NCM, and chemotherapy and radiation therapies are not effective in modifying the disease course.
Therapies to help manage neurological symptoms associated with the disorder include ventriculoperitoneal shunts to alleviate intracranial pressure in cases of hydrocephalus, and anticonvulsant medications to reduce seizures.
New targeted therapies are being explored, including inhibitors specific for Kit-ligand, hepatocyte growth factor, or the MET signalling pathway.
Drugs that block the MAPK (mitogen-activated protein kinase) pathway through MEK inhibition in cases of NCM have shown potential promising effects.
How do you prevent neurocutaneous melanosis?
While there are no specific preventive measures for NCM, recommended screening protocols for patients with congenital melanocytic naevi can play a pivotal role in early detection and improved monitoring of the condition. In patients with neurological symptoms or asymptomatic patients with at least one risk factor (eg, multiple satellite lesions; and/or a large-sized CMN, especially on the posterior axis), an MRI should be considered prior to 4–6 months of age for the highest sensitivity.
What is the outcome for neurocutaneous melanosis?
Prognosis for patients with neurological signs and symptoms is much worse than for those without. Those that remain asymptomatic have a normal life expectancy.
More than 50% of symptomatic patients will pass away within three years of symptom onset.
The mortality rate of melanoma arising in the CNS is almost 100%.
Bibliography
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Philadelphia: Elsevier; 2018.
- Gocmen R, Guler E, Arslan EA. A case of neurocutaneous melanosis and neuroimaging findings. J Radiol Case Rep. 2015;9(3):1–6. doi: 10.3941/jrcr.v9i3.2141 Article
- Jahnke MN, O'Haver J, Gupta D, et al. Care of Congenital Melanocytic Nevi in Newborns and Infants: Review and Management Recommendations. Pediatrics. 2021;148(6):e2021051536. doi: 10.1542/peds.2021-051536. Journal
- Jakchairoongruang K, Khakoo Y, Beckwith M, Barkovich AJ. New insights into neurocutaneous melanosis. Pediatr Radiol. 2018;48(12):1786–1796. doi: 10.1007/s00247-018-4205-x. Journal
- Kadonaga JN, Frieden IJ. Neurocutaneous melanosis: definition and review of the literature. J Am Acad Dermatol. 1991;24:747–755.
- Kinsler VA, Thomas AC, Ishida M, et al. Multiple congenital melanocytic nevi and neurocutaneous melanosis are caused by postzygotic mutations in codon 61 of NRAS. J Invest Dermatol. 2013;133(9):2229–2236. doi: 10.1038/jid.2013.70. Published correction appears in J Invest Dermatol. 2016;136(11):2326. Journal
- Küsters-Vandevelde HV, Willemsen AE, Groenen PJ, et al. Experimental treatment of NRAS-mutated neurocutaneous melanocytosis with MEK162, a MEK-inhibitor. Acta Neuropathol Commun. 2014;2:41. doi: 10.1186/2051-5960-2-41. Journal
- Moreira BL, Grunewald T, Côrtes AA, et al. Neurocutaneous melanosis. Radiol Bras. 2016;49(6):412–413. doi: 10.1590/0100-3984.2015.0128. Article
- Navarro-Fernandez IN, Mahabal GD. Congenital Nevus. In: StatPearls. Treasure Island (FL): StatPearls Publishing; July 2023. Available here
- Pawlikowski JS, Brock C, Chen SC, et al. Acute Inhibition of MEK Suppresses Congenital Melanocytic Nevus Syndrome in a Murine Model Driven by Activated NRAS and Wnt Signaling. J Invest Dermatol. 2015;135(8):2093–2101. doi: 10.1038/jid.2015.114. Published correction appears in J Invest Dermatol. 2015 Nov;135(11):2902. Journal
- Takayama H, Nagashima Y, Hara M, et al. Immunohistochemical detection of the c-met proto-oncogene product in the congenital melanocytic nevus of an infant with neurocutaneous melanosis. J Am Acad Dermatol. 2001;44(3):538-40. doi: 10.1067/mjd.2001.112403. Journal
- Viana AC, Gontijo B, Bittencourt FV. Giant congenital melanocytic nevus. An Bras Dermatol. 2013;88(6):863–878. doi: 10.1590/abd1806-4841.20132233. Published correction appears in An Bras Dermatol. 2014;89(1):190. Article
On DermNet
Other websites
-
Brock Elbank’s congenital melanocytic naevus (CMN) photography series: HOW DO YOU C ME NOW? — Hasselblad
