Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Congenital melanocytic naevus — extra information
Congenital melanocytic naevus
Author: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, 2001. Updated by Giri Raj, Dermatologist, New Plymouth; Dr Amanda Oakley, June 2014; with minor update September 2024.
Introduction
Classification
Prevalence
Clinical features
Symptoms
Causes
Diagnosis
Treatment
Other treatment options
What is a congenital melanocytic naevus?
A congenital melanocytic naevus (American spelling nevus) is a proliferation of benign melanocytes that are present at birth or develop shortly after birth. This form of a congenital naevus is also known as a brown birthmark.
Similar melanocytic naevi, or moles that were not present at birth, are often called ‘congenital melanocytic naevus-like’ naevi, ‘congenital type’ naevi or ‘tardive’ naevi.
Naevi may also form from other skin cells (eg, vascular naevi, which are formed from blood vessels). Some of these are also congenital (present at birth) and are described on other pages of DermNet.
Classification of congenital melanocytic naevi
Congenital melanocytic naevi are usually classified by their size in an adult. There are several different classifications.
- A small congenital melanocytic naevus is < 1.5 cm in diameter.
- A medium congenital melanocytic naevi is 1.5–19.9 cm.
- A large or giant congenital melanocytic naevus is ≥ 20 cm in diameter.
A modification of the above criteria is used in some centres in an effort to increase the accuracy of classification.
- Small congenital melanocytic naevi are < 1.5 cm in diameter.
- Medium congenital melanocytic naevi are 1.5–10 cm.
- Large congenital melanocytic naevi are between 11–20 cm.
- Giant congenital naevi are >20cm in diameter, and are further subdivided into:
- G1 (21–30 cm)
- G2 (31–40 cm)
- G3 (> 40 cm).
2013 Classification of congenital melanocytic naevi
In 2013, a new categorisation of congenital melanocytic naevi using predicted adult size was proposed:
- Small (< 1.5 cm)
- Medium (M1: 1.5–10 cm, M2: > 10–20 cm)
- Large (L1: > 20–30 cm, L2: > 30–40 cm)
- Giant (G1: > 40–60 cm, G2: > 60 cm)
- Satellite naevi: none, 1–20, > 20–50, and > 50 satellites.
Congenital melanocytic naevi should be described according to their body site, colours, surface features and whether or not there is hypertrichosis (hairs).
Progression over time
Congenital melanocytic naevi usually grow proportionally with the child. As a rough guide, the likely adult size of a congenital naevus can be calculated as follows:
- Lower limbs: adult size is x 3.3 size at birth
- Upper limbs/torso: adult size is x 2.8 size at birth
- Head: adult size is x 1.7 size at birth.
Descriptive names of some congenital naevi
Some congenital naevi are given specific descriptive names. Some of these are listed here.
- Also called naevus spilus
- Dark spots on a flat tan background
- The number of spots may increase or decrease over time
Satellite lesions
- Found on the periphery of central congenital melanocytic naevus or elsewhere on the body
- Smaller melanocytic naevi similar in appearance to
- Present in > 70% of patients with a large congenital melanocytic naevus
Tardive naevus
- Melanocytic naevus that appears after birth
- Slower growth and less synthesis of melanin than congenital naevus
- Histopathology is similar to true congenital melanocytic naevi
Garment naevus
- The name relates to the anatomical location of naevus
- Bathing trunk naevus involves central areas usually covered by a bathing costume, for example, buttocks
- Coat sleeve naevus involves an entire arm and proximal shoulder
- Affects some congenital and tardive melanocytic naevi
- Surrounding skin becomes lighter or white
- The central lesion may also become lighter and smaller and may disappear
- Due to immune destruction of melanocytes
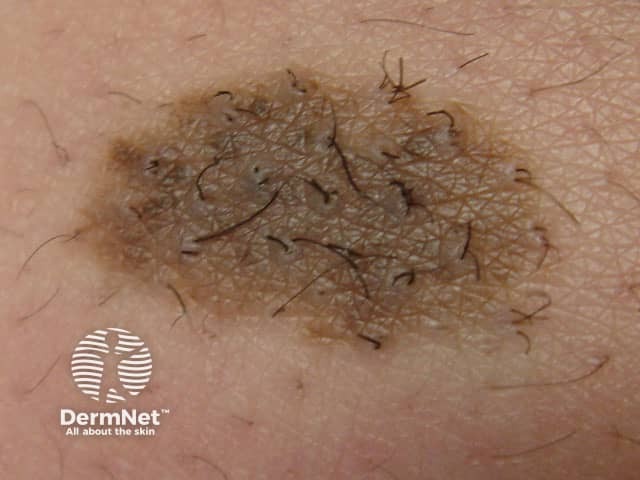
Congenital naevus
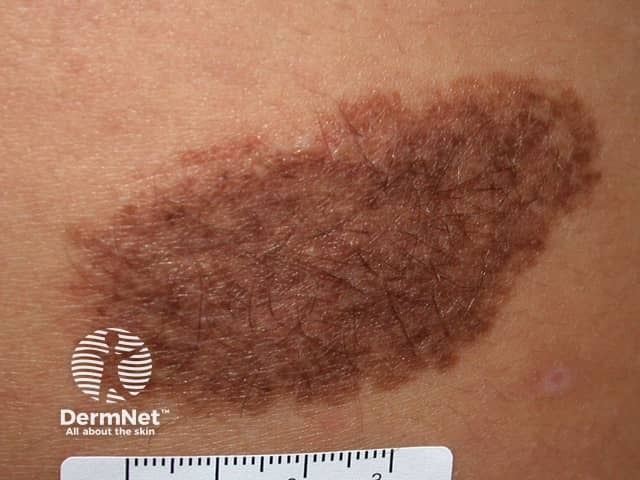
Congenital naevus
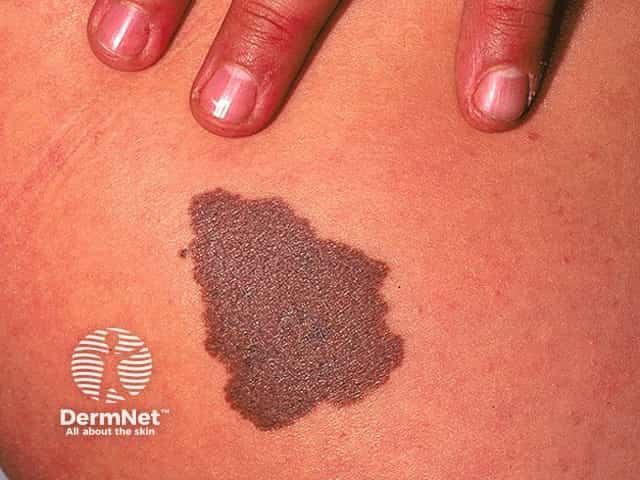
Congenital naevus
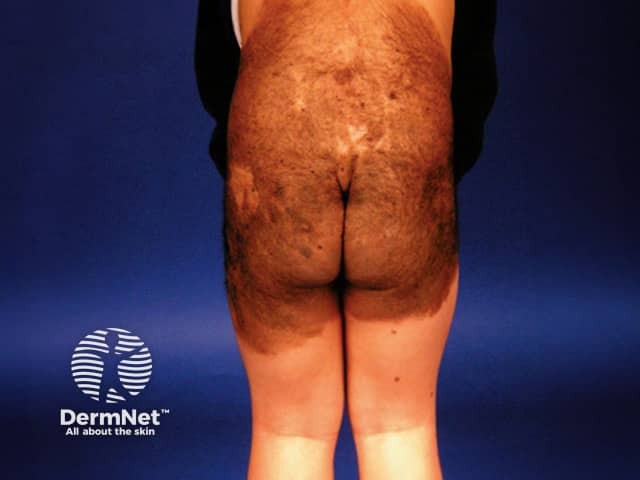
Congenital naevus
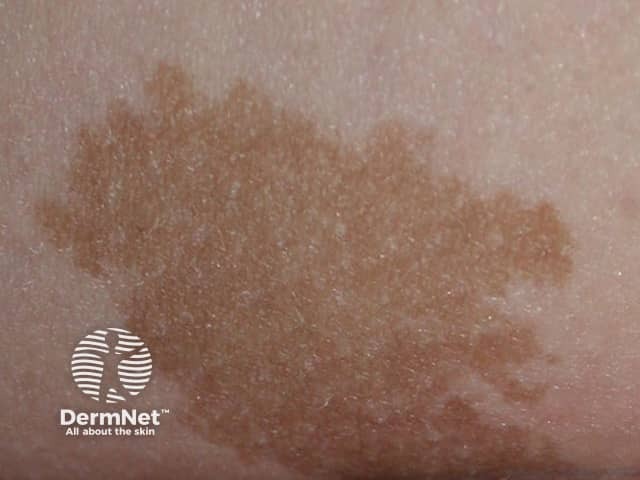
Congenital naevus
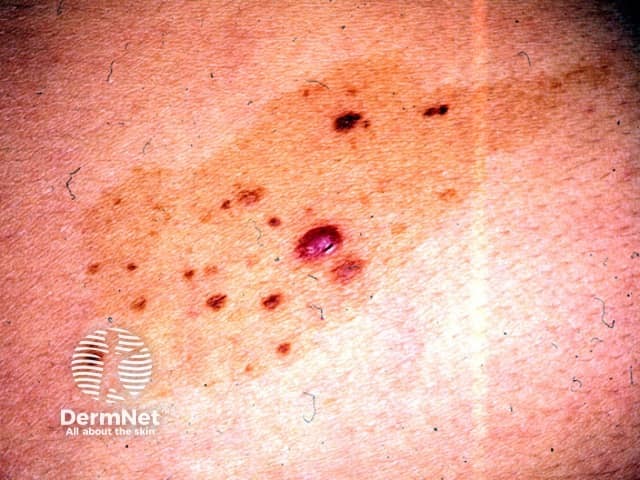
Congenital naevus
How common are congenital melanocytic naevi?
- Small congenital naevi occur in 1 in 100 births
- Medium congenital naevi occur in 1 in 1000 births
- Giant congenital melanocytic naevi are much rarer (1 in 20,000 live births)
They occur in all races and ethnic groups, and males and females are at equal risk.
What do congenital melanocytic naevi look like?
Congenital melanocytic naevi present as single or multi-shaded, round or oval-shaped pigmented patches. They may have increased hair growth (hypertrichosis). The surface may be slightly rough or bumpy.
Congenital naevi usually enlarge as the child grows but they may sometimes become smaller and less obvious with time. Rarely some may even disappear. However, they may also become darker, raised, more bumpy and hairy, particularly around the time of puberty.
Do congenital melanocytic naevi cause any symptoms?
Congenital melanocytic naevi are usually asymptomatic, however, some may be itchy, particularly larger lesions. It is thought there may be a reduced function of sebaceous (oil) and eccrine (sweat) glands, which may result in skin dryness and a heightened sensation of itch.
The overlying skin may become fragile and erode or ulcerate. Deep nests of melanocytes in the dermis may weaken the bonds between the epidermis and the dermis and account for skin fragility .
Congenital melanocytic naevi are often unsightly, especially when extensive, ie large or giant congenital melanocytic naevi. They may, therefore, result in anxiety and impaired self-image, especially when the lesions are in visible areas.
Giant melanocytic naevi, and to a lesser degree small lesions, are associated with increased risk of developing cutaneous melanoma, neurocutaneous melanoma and rarely other tumours (see below).
What causes a congenital melanocytic naevus?
Congenital melanocytic naevi are caused by localised genetic abnormalities resulting in the proliferation of melanocytes; these are cells in the skin responsible for normal skin colour. This abnormal proliferation is thought to occur between the 5th and 24th weeks of gestation. If proliferation starts early in development, giant and medium-sized congenital melanocytic naevi are formed. Smaller congenital melanocytic naevi are formed later in development after the melanoblasts (immature melanocytes) have migrated from the neural crest to the skin.
In some cases, there is also overgrowth of hair-forming cells and epidermis, forming an organoid naevus.
Very early onset of congenital naevus before the separation of the upper and lower eyelids results in kissing naevi, ie one part of the naevus is on the upper lid and the other part is on the lower eyelid.
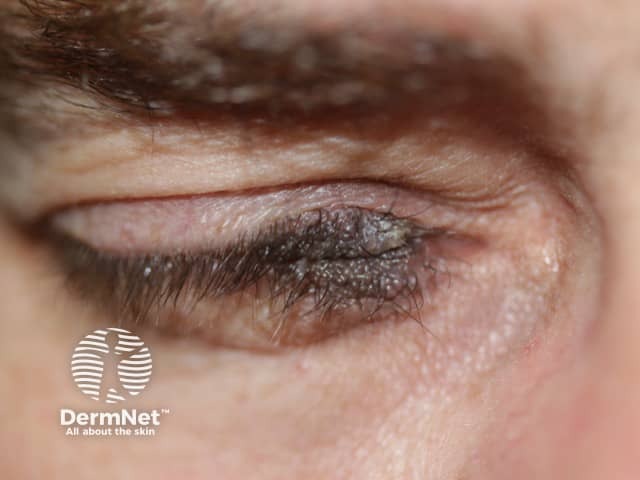
Kissing naevus
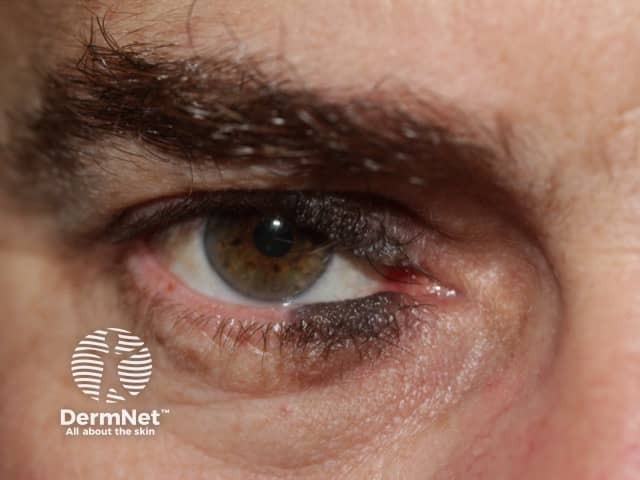
Kissing naevus
Molecular changes
Proto-oncogenes c-met and c-kit have important roles in the development of melanocytes. Hepatocyte growth factor, a cytokine (messenger protein) that regulates the proliferation and migration of melanocytes, may also be important in the development of congenital melanocytic naevi.
Neurocutaneous melanosis
Neurocutaneous melanosis is a rare syndrome defined by the proliferation of melanocytes in the central nervous system (brain and spinal cord) and the presence of a congenital melanocytic naevus. The majority of cases are associated with a giant congenital melanocytic naevus and satellite lesions.
The estimated risk of NCM developing in individuals with large and giant congenital melanocytic naevus (CMN) is 10–33%. However, it is likely that the majority of cases remain asymptomatic, and the true incidence remains unknown. The melanocytes in the brain and spinal cord may often be detected by an MRI scan.
Neurocutaneous melanocytosis may present with symptoms of raised intracranial pressure, such as:
- A headache
- Vomiting
- Irritability
- Focal cranial nerve signs
- Seizures
- Hydrocephalus
- Delayed development.
How is the diagnosis of congenital melanocytic naevus made?
The diagnosis of a congenital melanocytic naevus is usually based on the clinical appearance. If there is any doubt, examining the lesion with dermoscopy or taking a sample of the lesion for histology (biopsy) may show characteristic microscopic features.
Dermoscopy
Evaluation of the congenital melanocytic naevus by dermoscopy will reveal the pattern of pigmentation and its symmetry or lack of symmetry. The most common global pattern of congenital or tardive melanocytic naevus is globular, but reticular, structureless and mixed patterns may occur. The naevus may have differing structures across the lesion, sometimes leading to overall asymmetry of the structure.
Pathology
Congenital melanocytic naevi are usually larger than acquired naevi (which are melanocytic naevi that appear after 2 years of age), and the naevus cells often extend deeper into the dermis, fat layer, and deeper structures. The naevus cells characteristically cluster around blood vessels, hair follicles, sebaceous and eccrine glands, and other skin structures. Congenital naevus cells tend to involve collagen bundles in the deeper layers of the skin more than is the case in an acquired naevus.
Risk of developing melanoma within a congenital melanocytic naevus
The following characteristics of congenital melanocytic naevus are associated with the increased risk of development of melanoma (a skin cancer).
- Large or giant size
- Axial or paravertebral location (crossing the spine)
- Multiple congenital satellite naevi
- Neurocutaneous melanosis.
The risk of melanoma is mainly related to the size of the congenital melanocytic naevus. Small and medium-sized congenital melanocytic naevi have a very small risk, well under 1%. Melanoma is more likely to develop in giant congenital naevi (lifetime estimates are 5–10% but may be overestimates). A review of 27 studies including over 11,000 patients with congenital naevi, the prevalence of melanoma was 1.84 overall, and 2.73% in those with large congenital naevi. Melanoma can start deep inside the naevus or within any neuromelanosis found in the brain and spinal cord. Very rarely, other tissues that contain melanocytes may also be a source of melanoma such as the gastrointestinal tract mucosa. In 24% of cases, the origin of the melanoma cannot be identified.
Melanoma associated with a giant congenital melanocytic naevus or neuromelanosis can be very difficult to detect and treat.
The risk of development of melanoma is greater in early childhood; 70% of melanomas associated with giant congenital melanocytic naevi are diagnosed by the age of ten years.
Rarely, other types of tumour may develop within giant congenital melanocytic naevi including benign tumours (lipomas, schwannomas) and other malignant tumours (including sarcomas).
Melanoma can also develop within a small congenital melanocytic naevus. This is rare and likely to occur on the periphery of the naevus during adult life.
Is regular follow-up recommended?
- It can be useful to have a close-up photograph of the congenital naevus with a ruler beside it to assess for changes in size.
- Digital surveillance using dermoscopic images (mole mapping) may also be helpful to detect changes in structure. However, such changes are normal in childhood and should not usually give rise to concern.
- It is advisable to continue neurodevelopmental observation in those at risk of neurocutaneous melanosis.
Prognosis of melanoma associated with congenital melanocytic naevus
Unfortunately, when a rare melanoma arises within a giant congenital melanocytic naevus, the prognosis is unfavourable. This is due to the deeper origin of the tumour rendering it more difficult to detect on clinical examination, resulting in a later stage at presentation. The deeper location also facilitates earlier spread through blood and lymph vessels. In 24% of cases, the melanoma has already spread to other sites (metastases) at the time of the first diagnosis.
Treatment of a congenital melanocytic naevus
Management of a congenital melanocytic naevus must take into account the age of the subject, the lesion size, the location and depth, and the risk of developing malignant change within the lesion.
Giant congenital melanocytic naevus
The only definite indication for surgery in a giant congenital melanocytic naevus is when melanoma develops within it.
Small congenital naevus
If a small congenital naevus is growing at the same rate as the child and is not changing in any other way, the usual practice is not to remove it until the child is old enough to co-operate with a local anaesthetic injection, usually around the age of 10 to 12 years. Even then, removal is not essential.
Reasons to consider surgical removal may include:
- Unsightly appearance
- Difficulty in observing the mole (eg, scalp, back)
- A recent change in the lesion (darkening, lumpiness, increasing size)
- Melanoma-like appearance (irregular shape, variegated colour).
Prophylactic surgical removal of a naevus
The following factors should be considered prior to prophylactic surgical removal of a naevus.
- Prophylactic excision of a small lesion may be delayed until an age when the patient is old enough to make an informed choice.
- Small or medium-sized congenital melanocytic naevi are at low risk for developing malignant change.
- Irregular, lumpy or thick lesions or lesions that are difficult to clinically assess may have a lower threshold for consideration of surgical excision, so as not to miss a melanoma.
- 50% of melanomas diagnosed in those with giant congenital melanocytic naevi occur at another body site such as within the central nervous system. Therefore surgical excision of the lesion may not eliminate the risk of melanoma.
- Large or giant melanocytic lesions may be too large to excise completely.
- Large lesions may require a skin flap or graft to close the surgical defect.
Complications of surgery
Complications that may occur after surgery include:
- Graft or flap failure
- Infection
- Wound breakdown
- Bleeding or haematoma
- Hypertrophic or keloid scar
- Irritable or itchy scar.
Other treatment options for a congenital melanocytic naevus
Dermabrasion
Dermabrasion can allow partial removal of a large congenital naevus; deeper naevus cells may persist. Dermabrasion may lighten the colour of the naevus but may not reduce hair growth within it. It can cause scarring.
Tangential (shave) excision
Tangential or shave excision uses a blade to remove the top layers of the skin (epidermis and upper dermis). This may reduce the pigmentation but the lesion may not be completely removed. Shave excision may result in significant scarring.
Chemical peels
Chemical peels using trichloroacetic acid or phenol may lighten the pigmentation of a superficial (surface) congenital naevus that is located in the upper layers of the skin.
Laser ablation
Laser treatment is considered if surgical intervention is not possible. They may result in lightening of the lesion. Suitable devices include:
- Ruby Q-switched laser
- Carbon dioxide resurfacing laser
Techniques that result in partial removal of a congenital naevus can make the lesion more difficult to assess during long-term surveillance.
References
- Viana ACL, Gontijo B, Bittencourt FV. Giant congenital melanocytic nevus. An Bras Dermatol. 2013; 88(6): 863–78. PubMed
- Eds. Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Elsevier Saunders; 2012. P 1871-6
- Kovalyshyn I, Braun R, Marghoob A. Congenital melanocytic naevi. Aust J Derm. 2009; 50: 231–40. PubMed
- Krengel S, Scope A, Dusza SW, Vonthein R, Marghoob AA. New recommendations for the categorization of cutaneous features of congenital melanocytic nevi. J Am Acad Dermatol. 2013 Mar;68(3):441–51. doi: 10.1016/j.jaad.2012.05.043. Epub 2012 Sep 13. PubMed PMID: 22982004.
- Tan S, Hu H, Li G, Zhao J, Wu D. Prevalence, incidence density and standardized morbidity rate of melanoma among patients with congenital melanocytic naevi: a systematic review. Clin Exp Derm 2024;49(8):765-773. Journal
On DermNet
- Congenital melanocytic naevi – pathology
- Moles
- Neurocutaemous melanosis
- Melanoma
- Mole mapping
- Benign melanocytic lesions – common skin lesions course
- Skin lesions, tumours and cancers
Other websites
- Nevus Support Australia Inc
- Nevus Outreach, Inc.
- Nevus Network
- Melanocytic nevi — Medscape Reference
