Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Key clinical-trial evidence for risankizumab — extra information
Key clinical-trial evidence for risankizumab
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet Editor in Chief: Adjunct A/Prof Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. August 2019.
Introduction UltIMMa-1 and UltIMMa-2 IMMhance study IMMvent study Future potential
Introduction
In March 2019, risankizumab (trade name SKYRIZI™; AbbVie Inc., North Chicago, IL, USA) received US Food and Drug Administration (FDA) approval for the treatment of moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy [1].
Risankizumab has also been approved for the treatment of psoriasis in both Japan and the European Union in March 2019, and in Australia in 2020 It is not currently available in New Zealand.
Risankizumab received FDA approval in the US based on data from a Phase III psoriasis clinical trial programme, which evaluated the safety and efficacy of risankizumab in adults with moderate-to-severe plaque psoriasis in four studies: UltIMMa-1, UltIMMa-2, IMMhance, and IMMvent [1,2].
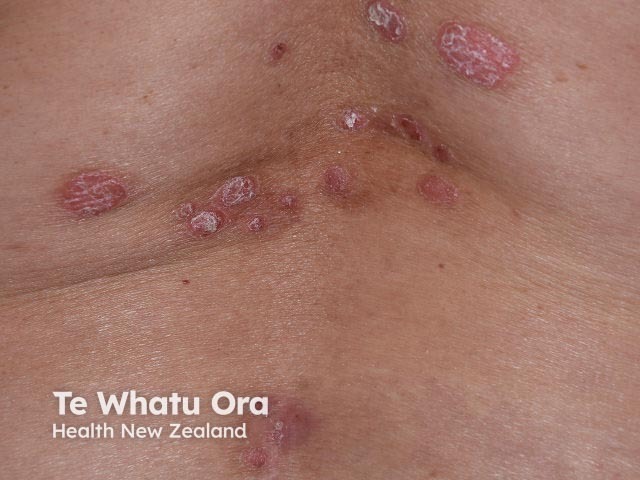
Chronic plaque psoriasis
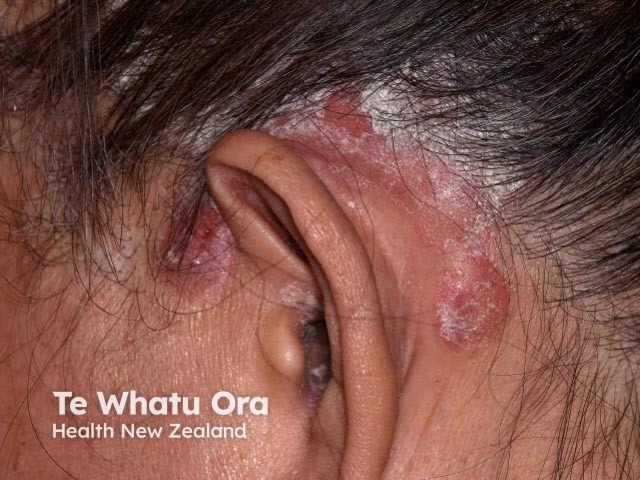
Chronic plaque psoriasis
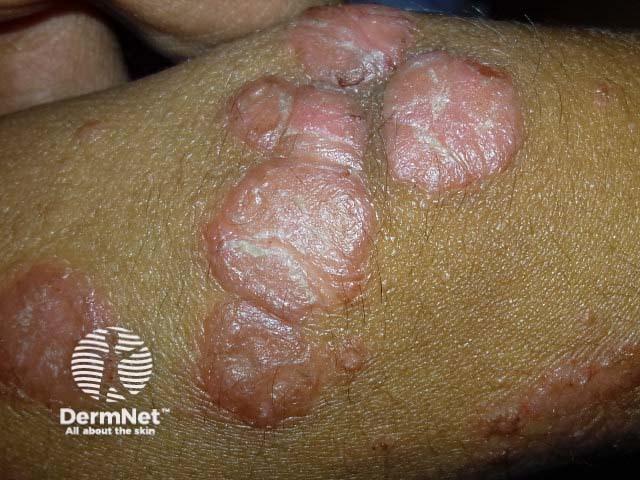
Chronic plaque psoriasis
UltIMMa-1 and UltIMMa-2 [3]
Study designs
UltIMMa-1 patients were randomly assigned to receive:
- Risankizumab 150 mg (n=304)
- Ustekinumab 45 mg or 90 mg (n=100)
- Placebo (n=102).
In UltIMMa-2, patients were randomly assigned to receive:
- Risankizumab 150 mg (n=294)
- Ustekinumab 45 mg or 90 mg (n=99)
- Placebo (n=98).
Following the 16-week double-blind treatment period (part A; Weeks 0–16), patients initially assigned to placebo switched to risankizumab 150 mg at Week 16, while the other patients continued their originally randomised treatment (part B; double-blind, Weeks 16–52). The study drug was administered subcutaneously at Weeks 0 and 4 during part A and at Weeks 16, 28, and 40 during part B.
The two primary endpoints were the proportions of patients achieving a 90% improvement in Psoriasis Area Severity Index (PASI 90) and a static Physician's Global Assessment (sPGA) score of 0 or 1 at Week 16. All efficacy analyses were done in the intention-to-treat patient population.
Results
Part A — Week 16 results
At Week 16 in UltIMMa-1, PASI 90 was achieved by 229 (75.3%) patients receiving risankizumab versus five (4.9%) receiving placebo and 42 (42.0%) receiving ustekinumab (p<0.0001 vs placebo and ustekinumab).
In UltIMMa-1, a sPGA 0 or 1 at Week 16 was achieved by 267 (87.8%) patients receiving risankizumab versus eight (7.8%) receiving placebo and 63 (63.0%) receiving ustekinumab (p<0.0001 vs placebo and ustekinumab).
At Week 16 in UltIMMa-2, PASI 90 was achieved by 220 (74.8%) patients receiving risankizumab versus two (2.0%) receiving placebo and 47 (47.5%) receiving ustekinumab (p<0.0001 vs placebo and ustekinumab) [3].
At week 16, 246 (83.7%) patients receiving risankizumab versus five (5.1%) receiving placebo and 61 (61.6%) receiving ustekinumab achieved a sPGA score of 0 or 1 (p<0.0001 vs placebo and ustekinumab).
The examination of age, gender, race, body weight, baseline PASI score, and previous treatment with systemic or biological agents did not identify differences in response to risankizumab among these subgroups at Week 16.
Part B — Week 52 results
At Week 52 in both UltIMMa-1 and UltIMMa-2, 82% and 81% of patients treated with risankizumab achieved PASI 90, respectively, and 56% and 60% achieved 100% improvement in PASI score (PASI 100), respectively (p<0.0001 vs placebo).
In both UltIMMa-1 and UltIMMa-2, most patients treated with risankizumab who achieved PASI 90 and PASI 100 at Week 16 maintained this response at Week 52 (88% and 80%, respectively).
Adverse reactions
Part A — Week 16 results
Overall in both UltIMMa-1 and UltIMMA-2, the most common adverse events associated with risankizumab treatment were:
- Upper respiratory infections (13%)
- Headache (3.5%)
- Fatigue (2.5%)
- Injection site reactions (1.5%)
- Tinea infections (1.1%).
In part A of UltIMMa-1, adverse events occurred in 49.7% of the patients on risankizumab, 50.0% on ustekinumab, and 51.0% on placebo.
In part A of UltIMMa-2, adverse events occurred in 45.6% of the patients on risankizumab, 53.5% on ustekinumab, and 45.9% on placebo.
Serious infections occurred in 0.3% in the risankizumab group and 3.0% in the ustekinumab group in UltIMMa-1 and in 1.0% in both risankizumab and ustekinumab groups in UltIMMa-2. Serious infections in the risankizumab group included cellulitis, osteomyelitis, sepsis, and herpes zoster.
Serious adverse events were reported in 2.3% of risankizumab-treated patients, 8.0% of ustekinumab-treated patients, and 2.9% of placebo-treated patients in UltIMMa-1, and in 2.0% of risankizumab-treated patients, 3.0% of ustekinumab-treated patients, and 1.0% of placebo-treated patients in UltIMMa-2.
Malignancy was reported in two patients on risankizumab (squamous cell carcinoma in UltIMMa-1; basal cell carcinoma in UltIMMa-2) and in one patient in the placebo group (squamous cell carcinoma in UltIMMa-1).
Of note, there were no events of tuberculosis, opportunistic infections, major adverse cardiovascular events (MACEs), or serious hypersensitivity across both studies.
Part B — Week 52 results
In part B of both UltIMMa-1 and UltIMMa-2, the most frequent adverse events were:
- Viral upper respiratory tract infection
- Upper respiratory tract infection
- Urinary tract infection
- Influenza
- Headache.
In UltIMMa-1, adverse events occurred in 61.3% of the patients continuing on risankizumab, 66.7% on ustekinumab, and 67.0% of the patients switching from placebo to risankizumab.
In UltIMMa-2, adverse events occurred in 55.7% of the patients continuing on risankizumab, 74.5% on ustekinumab, and 64.9% of the patients switching from placebo to risankizumab.
During part B, serious infections occurred in 0.7% of the patients on risankizumab in both UltIMMa-1 and UltIMMa-2, and in 1.0% of the patients on ustekinumab and the patients switching from placebo to risankizumab in UltIMMa-2.
Serious adverse events in UltIMMa-1 were reported in 5.4% of risankizumab-treated patients, 4.0% of ustekinumab-treated patients, and 3.1% of the patients switching from placebo to risankizumab.
In UltIMMa-2, serious adverse events were reported in 4.5% of risankizumab-treated patients, 4.3% of ustekinumab-treated patients, and 3.2% of the patients switching from placebo to risankizumab.
Through to Week 52, two patients on risankizumab had MACEs, including one patient with cardiovascular death and one patient with type 1 myocardial infarction. Malignancy was reported in one patient on continuous risankizumab (basal cell carcinoma in UltIMMa-2), in one patient on ustekinumab (prostate cancer in UltIMMa-2), and in two patients switching from placebo to risankizumab (one patient with both basal cell and squamous cell carcinoma in UltIMMa-1; one patient with breast cancer in UltIMMa-2).
Immunogenicity
By Week 52 in both studies, approximately 24% (263/1079) of patients treated with risankizumab at the recommended dose had developed antibodies to risankizumab.
Of these patients, approximately 57% (14% of all subjects treated with risankizumab) had antibodies that were classified as neutralising. The higher antibody titres seen in approximately 1% of subjects treated with risankizumab were associated with lower risankizumab concentrations and reduced clinical response [1,3].
IMMhance study [4]
Study design
IMMhance was a Phase III multicentre randomised, double-blind, placebo-controlled study to evaluate the impact of treatment withdrawal and re-treatment with risankizumab compared to placebo in adult patients with moderate-to-severe plaque psoriasis.
IMMhance enrolled 507 patients (407 randomised to risankizumab 150 mg and 100 randomised to placebo). Patients received treatment at Weeks 0, 4, and every 12 weeks thereafter.
Results
At Week 16, risankizumab was superior to placebo for the co-primary endpoints of a sPGA score of 0 or 1 (84% risankizumab and 7% placebo) and PASI 90 (73% risankizumab and 2% placebo).
The following endpoints had the respective response rates at Week 16:
- A sPGA score of 0: 46% with risankizumab and 1% with placebo
- PASI 100: 47% with risankizumab and 1% with placebo
- 75% improvement in PASI score (PASI 75): 89% with risankizumab and 8% with placebo.
Patients who were originally on risankizumab and had a sPGA score of 0 or 1 at Week 28 were re-randomised to continue risankizumab every 12 weeks or withdrawal of therapy. At Week 52, 87% (97/111) of the patients re-randomised to continue treatment with risankizumab had a sPGA score of 0 or 1 compared to 61% (138/225) who were re-randomised to the withdrawal of risankizumab.
Adverse events
The safety profile was consistent with previously reported Phase III trials with no new safety signals detected.
Through to Week 16, serious adverse effects occurred in 2% of patients in the risankizumab group and 8% of patients in the placebo group. In the second part of the study (post-Week 28), serious adverse effects occurred in 6% of patients re-randomised to the risankizumab group and 6% of patients re-randomised to the placebo group.
One patient receiving risankizumab had intestinal adenocarcinoma and metastatic hepatic cancer and died, while a second patient treated with risankizumab died due to an unknown cause that was considered as a MACE.
There were two additional adjudicated MACE cases: the first event occurred in the placebo arm in the first phase of the study while the second occurred in the risankizumab arm in the second phase. All three patients with a MACE had a previous history of cardiovascular risk factors.
IMMvent study [5]
IMMvent was a Phase III, randomised, double-blind, double-dummy, active-controlled study to evaluate the efficacy and safety of risankizumab compared to adalimumab in adult patients with moderate-to-severe plaque psoriasis over 44 weeks.
Study design
Patients were randomised 1:1 to receive risankizumab 150 mg (Weeks 0, 4, 16, and 28) or adalimumab (80 mg at Week 0; 40 mg every other week from Week 1).
Adalimumab-treated patients who achieved from 50% improvement in PASI score (PASI 50) to < PASI 90 at Week 16 were re-randomised 1:1 to either continue adalimumab or switch to risankizumab (Weeks 16, 20, and 32).
Results
At Week 16, risankizumab-treated patients achieved significantly higher PASI 75 (90.7% vs 71.7%), PASI 90 (72.4% vs 47.4%), PASI 100 (39.9% vs 23.0%), and sPGA score of 0 or 1 (83.7% vs 60.2%) response rates compared with patients treated with adalimumab.
Among adalimumab-treated patients with PASI 50 to < PASI 90 responses at Week 16, a significantly higher proportion of patients switching to risankizumab achieved PASI 90 (66.0% vs 21.4%) and PASI 100 (39.6% vs 7.1%) responses at Week 44 compared with patients continuing on adalimumab.
Among adalimumab-treated patients who achieved from PASI 50 to < PASI 90 at Week 16, PASI 90 and PASI 100 responses were significantly higher in patients who switched to risankizumab, starting at Week 20, compared with patients who continued on adalimumab.
Adverse reactions
The rates of treatment-emergent adverse events leading to discontinuation were generally low and were comparable between patients continuing on adalimumab and patients switching from adalimumab to risankizumab. A similar safety profile was observed between patients continuing risankizumab and patients switching directly from adalimumab to risankizumab.
What is the future potential for risankizumab?
Risankizumab offers remarkable rates of complete or near-complete clearance of skin disease, along with a good safety profile so far and an excellent dosing regimen. The selective inhibition of the p19 subunit of interleukin (IL)-23 may present several advantages compared to the inhibition of p40 subunit shared by IL-23 and IL-12 and even a downstream inhibition of IL-17 or its receptor.
Overall, risankizumab showed a similar safety and tolerability profile to ustekinumab and adalimumab but superior efficacy. Future studies with more patients and longer follow-up periods will help establish a more complete safety profile.
More data from clinical trials comparing risankizumab with other IL-23 and IL-17 inhibitors will be essential to establish the value of risankizumab in the treatment of psoriasis.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- US Food and Drug Administration. SKYRZI™ (risankizumab-rzaa) injection, for subcutaneous use. US prescribing information. April 2019. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf (accessed 30 August 2019)
- Medscape. Risankizumab (Rx). Drugs & Diseases. Available at: http://reference.medscape.com/drug/skyrizi-risankizumab-1000307 (accessed 30 August 2019).
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018; 392: 650–61. DOI: 10.1016/S0140-6736(18)31713-6. PubMed
- Blauvelt A, Papp K, Gooderham M, et al. Efficacy and safety of risankizumab, an interleukin-23 inhibitor, in patients with moderate-to-severe chronic plaque psoriasis: 16-week results from the phase III IMMhance trial. Br J Dermatol 2017; 177(5): E248.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet 2019: 394: 576–86. DOI: 10/1016/S0140-6736(19)30952-3. PubMed
On DermNet
- Risankizumab
- Guidelines for the management of psoriasis
- Treatment of psoriasis
- PASI score
- Psoriasis PO-PASI scoring
- Chronic plaque psoriasis
- Biological agents for psoriasis
Other websites
- Risankizumab for treating moderate to severe plaque psoriasis — British Association of Dermatology
