Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Ustekinumab for psoriasis — extra information
Treatments Autoimmune/autoinflammatory
Ustekinumab for psoriasis
Author: Anoma Ranaweera, Medical writer, 2011. DermNet Editor in Chief: Adjunct A/Prof Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand. Reviewed and revised April 2019.
Introduction
How it works
Dosage
Adverse events
Precautions
Vaccinations
Drug interactions
Use of ustenkinumab
Monitoring
What is ustekinumab?
Ustekinumab (brand name STELARA™) is a biological treatment used to treat moderate to severe psoriasis. It is a human monoclonal antibody that antagonises interleukin-12 (IL-12) and IL-23. Good to excellent responses are seen in more than two-thirds of patients with chronic plaque psoriasis treated with ustekinumab.
Ustekinumab has been shown in small studies to be useful in other forms of psoriasis, including nail psoriasis, erythrodermic psoriasis, generalised pustular psoriasis and palmoplantar pustulosis. It is also used to treat psoriatic arthritis and Crohn disease.
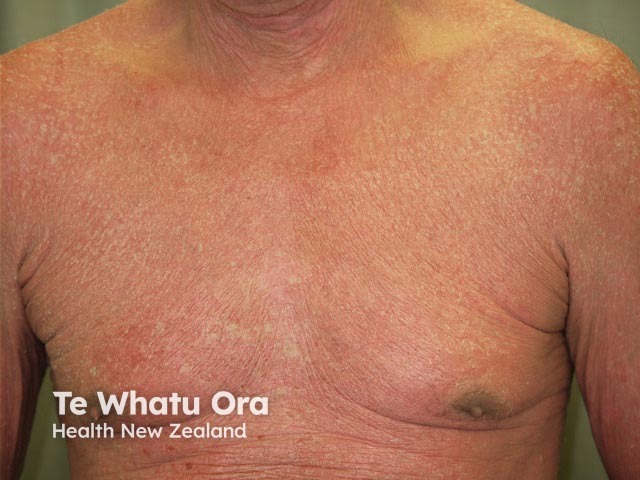
Erythrodermic psoriasis before ustekinumab
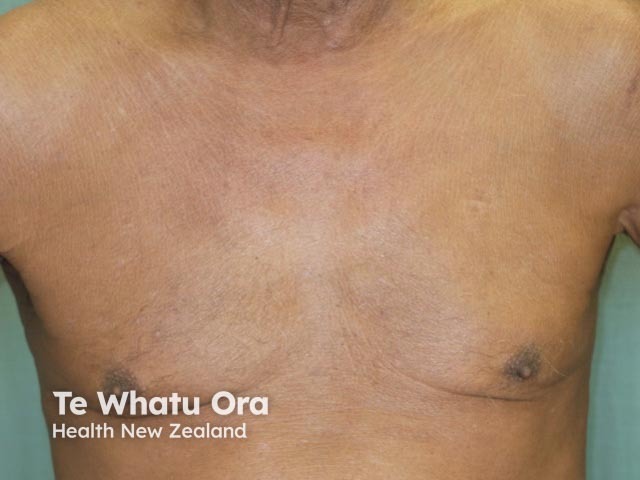
Erythrodermic psoriasis 2 weeks after first dose of ustekinumab
How does ustekinumab work in psoriasis?
Psoriasis is caused by an increase in the production of T-cells and is influenced by cytokines, the chemical messengers produced by cells. Certain cytokines cause skin cells to grow rapidly, producing plaques of psoriasis. Ustekinumab is a monoclonal antibody that targets the p40 subunit of the cytokines IL-12 and IL-23, preventing them from binding and activating T-lymphocytes.
Dosing of ustekinumab
Ustekinumab is given by subcutaneous injection. The second dose is given 4 weeks after the first injection, and further doses are delivered every 12 weeks. It reaches its peak effect at around 28 weeks.
- The dose for patients weighing less than 100 kg is 45 mg
- The dose for patients weighing more than 100 kg is 90 mg
- Each injection is best administered at a different location (such as upper arms, buttocks, thighs, or abdomen)
Adverse events due to ustekinumab
To date, adverse infections are consistent with that seen with other biologics.
- Infections – ustekinumab may increase the risk of infections and reactivation of latent infections. Serious bacterial, fungal, and viral infections have been observed in subjects receiving the drug.
- Malignancies – ustekinumab is an immunosuppressant and may increase the risk of malignancy (cancer) including skin cancers.
- Immune system disorders have been reported during post-approval use with ustekinumab. These have included serious allergic reactions (including angioedema, breathlessness and low blood pressure) and hypersensitivity (allergy) reactions (including rash and urticaria).
- Injection site reactions include pain, swelling, itch, thickening, bleeding, and bruising.
Precautions when considering ustekinumab
- Chronic infection or a history of recurrent infection. Ustekinumab should not be administered until the infection resolves or is adequately treated.
- Patients treated with ustekinumab may be susceptible to infection (including tuberculosis, atypical mycobacteria), salmonella (including nontyphi strains), and Bacillus Calmette-Guerin (BCG) vaccinations.
- Ustekinumab should not be administered to patients with active tuberculosis. Prior to biologic treatment, chest X-ray, Mantoux and/or QuantiFERON gold indirect testing should be performed. Consider anti-tuberculosis therapy prior to initiation of ustekinumab in patients with a past history of latent or active tuberculosis. Patients receiving ustekinumab should be monitored closely for signs and symptoms of active tuberculosis during and after treatment.
Vaccinations and ustekinumab
Immunisation status should be reviewed prior to starting ustekinumab. If necessary, vaccines should be updated prior to treatment with a biologic agent. Annual influenza vaccination is recommended.
As they may induce illness in immunodeficient individuals, live vaccines should not be used during treatment with ustekinumab. Currently, available live attenuated viral vaccines include measles, mumps, rubella, varicella, yellow fever, influenza (intranasal vaccine) and the oral polio vaccine. Live attenuated bacterial vaccines include BCG and oral typhoid vaccine.
Read more about immunisation in immunosuppressed dermatology patients.
Drug interactions with ustekinumab
- No formal drug-drug interaction studies have been conducted with ustekinumab.
- The safety of ustekinumab in combination with immunosuppressive agents or phototherapy has not been evaluated.
Use of ustekinumab in specific populations
Pregnancy Category B
There are no studies of ustekinumab in pregnant women. Ustekinumab should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing mothers
Caution should be exercised when ustekinumab is administered to a nursing woman. The unknown risks to the infant from gastrointestinal or systemic exposure to ustekinumab should be weighed against the known benefits of breastfeeding.
Paediatric use
A study has shown the safety and effectiveness of ustekinumab in children aged 12 to 17 years to be similar to that in adults.
Hepatic and renal impairment
No pharmacokinetic data are available in patients with liver or kidney disease treated with ustekinumab.
Monitoring while on ustekinumab
It is recommended that patients on biologic medications have routine blood tests every 6 months or so, including full blood count and liver function tests. TB testing should be repeated from time to time.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Molina-Leyva A, Husein-Elahmed H, Naranjo-Sintes R, Ruiz-Carrascosa JC. Safety and effectiveness of ustekinumab for treatment of moderate to severe psoriasis: a prospective study in a clinical setting. J Drugs Dermatol. 2014 Aug 1;13(8):971–4. PubMed PMID: 25116977.
- Meng Y, Dongmei L, Yanbin P, Jinju F, Meile T, Binzhu L, Xiao H, Ping T, Jianmin L. Systematic review and meta-analysis of ustekinumab for moderate to severe psoriasis. Clin Exp Dermatol. 2014 Aug;39(6):696–707. doi: 10.1111/ced.12390. PubMed PMID: 25039593.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic Efficacy of Interleukin12/Interleukin 23 Blockade in Generalized Pustular Psoriasis Regardless of IL36RN Mutation Status. JAMA Dermatol. 2016. PubMed
On DermNet
- Ustekinumab trials
- Biological agents for psoriasis
- Biological treatments
- Psoriasis
- Acitretin
- Methotrexate
- Ciclosporin
- Biologics and reproduction in psoriasis
- Immunisation in immunosuppressed dermatology patients
- Monitoring immune-modulating drugs used in dermatology
- Tuberculosis screening for patients prescribed immunosuppressing drugs
- Drug-induced psoriasis
Other websites
- Stelara solution for injection — Consumer Medicine Information, Medsafe New Zealand
- Stelara solution for injection — Manufacturer Data Sheet, Medsafe New Zealand
- Ustekinumab: differential use in psoriasis — Open Access article, Clinical, Cosmetic and Investigational Dermatology
- Ustekinumab — British Association of Dermatologists
