Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Clascoterone — extra information
Treatments Follicular disorder
Clascoterone
December 2022
Author: Dr Jacqueline K Nguyen, Resident, St Vincent's Hospital, Australia (2022).
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction Uses How it works Dosage and administration Benefits Disadvantages Contraindications
What is clascoterone?
Topical clascoterone cream 1% (Winlevi®) is a novel androgen receptor inhibitor used for the treatment of acne vulgaris in patients aged 12 years and older.
Clascoterone was approved by the US Food and Drug Administration (FDA) in August 2020 for the treatment of acne vulgaris, but is not yet available in some countries including Australia and New Zealand at the time of writing.
What is clascoterone used for?
Acne vulgaris
There have been several trials to date demonstrating the efficacy and safety of clascoterone cream 1% for moderate-to-severe acne vulgaris.
- A pilot study (Trifu et al. 2011) found that clascoterone cream 1% was significantly better than placebo in reducing inflammatory lesion count, total lesion count, and acne severity index in men with facial acne.
- They also found clascoterone to be more effective (but not reaching statistical significance) in reducing total lesion count and acne severity index when compared to tretinoin 0.05% cream.
- Two landmark phase III trials (CB-03-01/25 and CB-03-01/26) with 1440 patients found that significantly more patients (aged 9 years and older) treated with clascoterone cream 1% achieved treatment success (as defined by ≥2/4 reduction in Investigator Global Assessment severity scores) compared to vehicle cream after 12 weeks (18.4–20.3% clascoterone group vs 6.5–9.0% vehicle group, p <0.001).
- Clascoterone was also associated with a statistically significant greater absolute reduction in lesion counts (inflammatory, non-inflammatory, and total) from baseline to 12 weeks compared to vehicle cream.
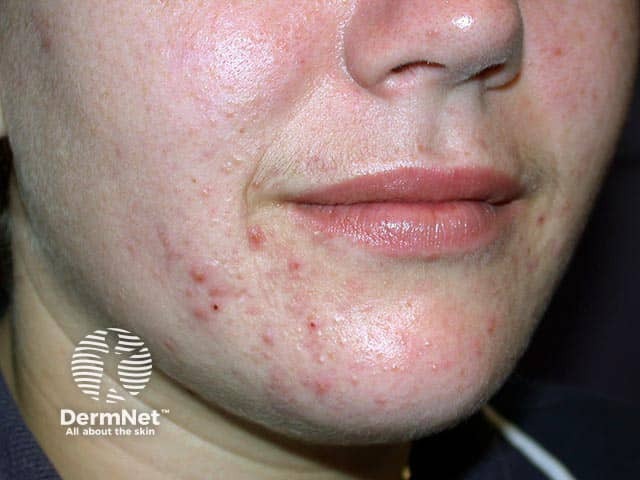
Papulopustular chin acne
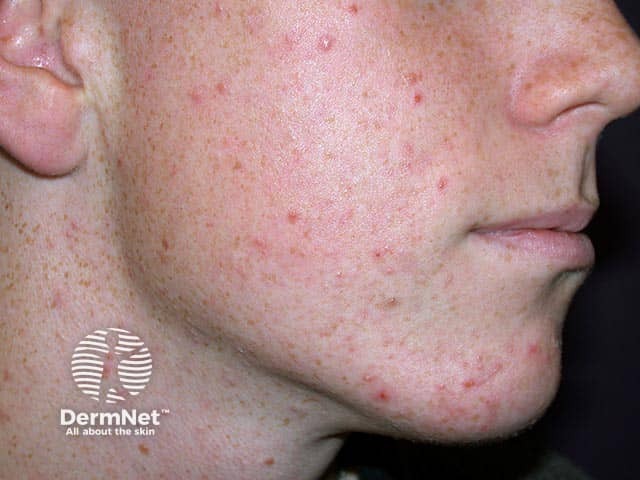
Mild papulopustular facial acne
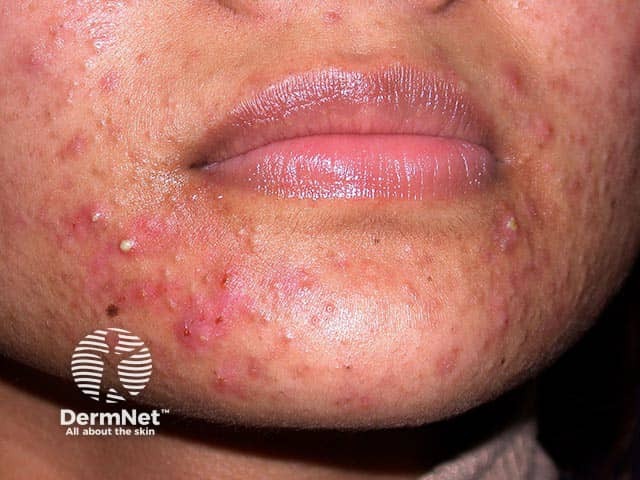
Mixed comedonal and inflammatory facial acne in skin of colour
Androgenetic alopecia
Clascoterone has also been suggested to be an effective treatment for androgenetic alopecia from preliminary studies, although it is not currently approved for use in this context.
The proposed mechanism of action is through antagonizing dihydrotesterone’s effects on dermal papilla cells, reducing the production of prostaglandin D2 and interleukin 6 (IL-6), regulating secretion of sebum, and reducing hair miniaturisation.
How does clascoterone work?
Clascoterone or cortexolone 17α-propionate is the first of its kind — a topical treatment targeting the hormonal pathogenesis of acne vulgaris.
It is postulated that clascoterone competes with androgens such as dihydrotestosterone (DHT) for androgen receptors, inhibiting the downstream signalling pathways of androgen receptor-regulated gene transcription in sebaceous glands and dermal papilla cells responsible for acne pathogenesis.
Dosage and administration
Clascoterone is currently available as a 1% (10mg/g) cream. A thin, uniform layer to the affected area twice a day: morning and evening is recommended.
What are the benefits of clascoterone?
Targeting androgen inhibition is a known effective treatment for acne vulgaris, as is seen in current utilisation of the anti-androgenic effects of spironolactone and some oral contraceptives. These, however, can cause systemic adverse effects and are also contraindicated in males.
Clascoterone 1% cream has selective topical activity, limiting systemic androgenic effects. Clascoterone is also highly potent, and found to be four times more potent than progesterone.
What are the disadvantages of clascoterone?
Side-effects and risks
The reported adverse effects of clascoterone cream 1% are mild and infrequent. Clascoterone has a similar safety profile to vehicle cream from existing studies.
Local skin reactions may include:
- Erythema
- Pruritus
- Scaling/dryness
- Oedema
- Skin atrophy
- Stinging/burning
- Striae rubrae
- Telangiectasia.
Reported treatment-emergent adverse events (<2%) included:
- Nasopharyngitis
- Headache
- Oropharyngeal pain.
Other considerations
- Paediatric patients may be more susceptible to systemic adverse effects.
- A pharmacokinetic study in 42 patients found hypothalamic-pituitary-adrenal (HPA) axis suppression at day 14 of treatment with 4g-6g BD of clascoterone. However, all patients returned to normal HPA axis 4 weeks after the end of the treatment.
- Hyperkalaemia was observed in some patients during the clinical trials (5% treated with clascoterone compared to 4% of vehicle-treated subjects).
What are the contraindications with clascoterone?
There are currently no known contraindications from the existing studies.
There is, however, a lack of safety evidence available for the use of clascoterone in patients less than 12 years of age, in pregnancy, during lactation (breastfeeding), and in those over 65 years of age.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
Bibliography
- Dhillon S. Clascoterone: First Approval. Drugs. 2020;80(16):1745–50. doi:10.1007/s40265-020-01417-6. Abstract
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and Safety of Topical Clascoterone Cream, 1%, for Treatment in Patients With Facial Acne: Two Phase 3 Randomized Clinical Trials. JAMA Dermatol. 2020;156(6):621–30. doi:10.1001/jamadermatol.2020.0465. Journal
- Mazzetti A, Moro L, Gerloni M, Cartwright M. A Phase 2b, Randomized, Double-Blind Vehicle Controlled, Dose Escalation Study Evaluating Clascoterone 0.1%, 0.5%, and 1% Topical Cream in Subjects With Facial Acne. J Drugs Dermatol. 2019;18(6):570. Journal
- Mazzetti A, Moro L, Gerloni M, Cartwright M. Pharmacokinetic Profile, Safety, and Tolerability of Clascoterone (Cortexolone 17-alpha propionate, CB-03-01) Topical Cream, 1% in Subjects With Acne Vulgaris: An Open-Label Phase 2a Study. J Drugs Dermatol. 2019;18(6):563. Journal
- Santhosh P, George M. Clascoterone: a new topical anti-androgen for acne management. Int J Dermatol. 2021;60(12):1561–5. doi:10.1111/ijd.15752. Abstract
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45(7):913–4. doi:10.1111/ced.14292. Journal
- Trifu V, Tiplica GS, Naumescu E, Zalupca L, Moro L, Celasco G. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. A pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165(1):177–83. doi:10.1111/j.1365-2133.2011.10332.x. Abstract
On DermNet
- Acne vulgaris
- Acne treatment
- Topical treatment for acne
- Male pattern hair loss
- Female pattern hair loss
- Anti-androgen therapy
Other websites
