Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Genetics of melanoma — extra information
Lesions (cancerous) Diagnosis and testing
Genetics of melanoma
Author: Anoma Ranaweera, DermNet Medical Writer. 2011.
What is melanoma?
What are genes?
Role of genes in melanoma
Risk of inheriting a mutated gene
Inherited gene mutations with melanoma risk
Non-inherited gene mutations with melanoma risk
Genetic testing
What is melanoma?
Melanoma is a potentially serious type of skin cancer due to uncontrolled growth of pigment cells, called melanocytes. Melanoma is most common in white-skinned individuals, but it may rarely develop in those with dark skin as well. About one in fifteen white-skinned New Zealanders are expected to develop melanoma in their lifetime — Australia and New Zealand have the highest reported rates of melanoma in the world.
A large number of genes are being investigated for their role in melanoma, including inherited genes and genetic defects that are acquired due to environmental factors, such as excessive sun exposure.
See also Genes and melanoma.
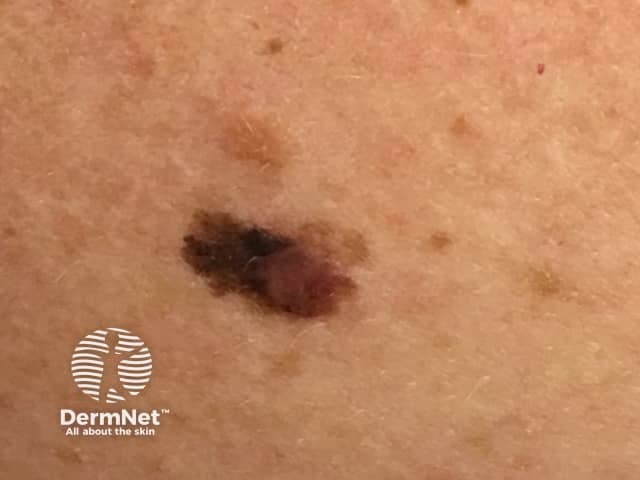
Superficial spreading melanoma
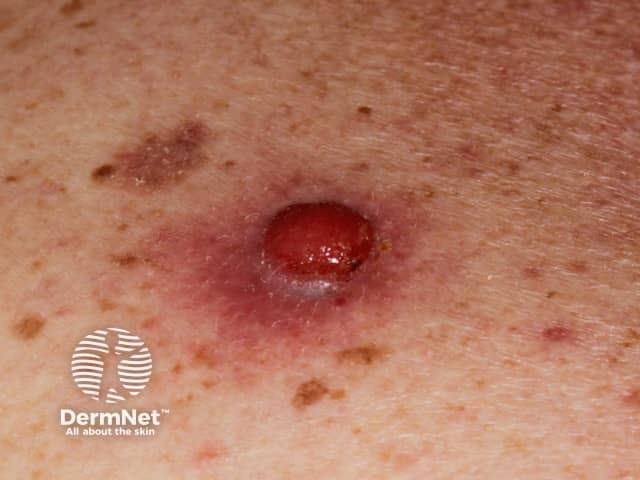
Amelanotic nodular melanoma*
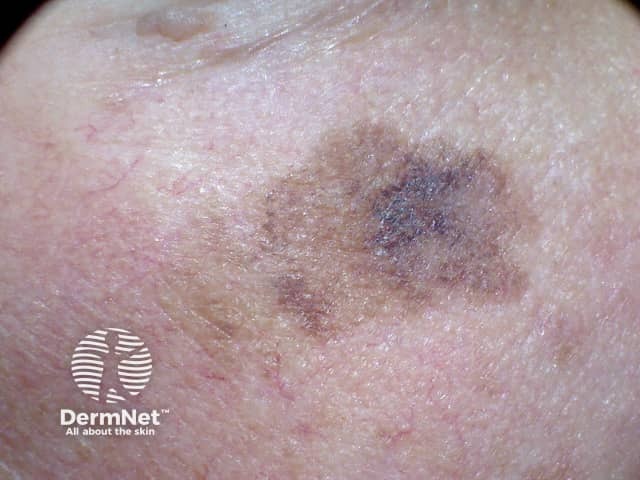
Lentigo maligna
Credit: Dr Eugene Tan.
What are genes?
Genes carry information in the form of DNA within each cell of the human body and are packaged onto chromosomes. There are 23 pairs of chromosomes in each cell. One chromosome of each pair is inherited from the person's father and one from the person's mother.
Genes control how the cell functions, including how quickly it grows, how often it divides, and how long it lives.
To control these functions, genes produce proteins that perform specific tasks and act as messengers for the cell. Therefore, it is essential that each gene has the correct instructions or 'code' for making its protein so that the protein can perform the proper function for the cell.
What role do genes play in melanoma?
Many cancers begin when one or more genes in a cell are mutated (changed), creating an abnormal protein.
A person may either be born with a genetic mutation in all of their cells (germline mutation) or acquire a genetic mutation in a single cell during his or her life sometimes as a result of exposure to environmental factors, such as UV radiation from the sun.
Most melanomas (about 90%) are considered sporadic, meaning that the damage to the genes occurs by chance after a person is born, and there is no risk of passing on the gene to a person's children.
An increased risk of melanoma occurs when specific gene mutations are passed within a family from generation to generation. Such inherited melanoma is sometimes called familial melanoma.
Inherited risk of melanoma is suspected if two or more first-degree relatives (parents, brothers, sisters) are diagnosed with melanoma.
Many people who have an increased risk of melanoma never develop the disease; only 10% of melanoma is familial.
What are the chances a mutated gene is inherited?
Every cell usually has two copies of each gene: one inherited from a person's mother and one inherited from a person's father.
Hereditary melanoma follows an autosomal dominant inheritance pattern, in which a mutation needs to happen in only one copy of the gene for the person to have an increased risk of getting the disease.
This means that a parent with a gene mutation may pass on a copy of the normal gene or a copy of the gene with a mutation. Therefore, a child who has a parent with a mutation has a 50% chance of inheriting that mutation. A brother, sister, or parent of a person who has a gene mutation also has a 50% chance of having the same mutation.
If a person has a first-degree relative with melanoma, his or her risk of developing melanoma is two to three times greater than the average risk. The risk is higher if several family members that live in different locations have been diagnosed with melanoma.
Most experts strongly recommend that people concerned about their family history of melanoma consult a genetic counsellor. Genetic counsellors are trained to assess the potential for hereditary cancer risk in a family and can identify appropriate genetic testing.
Which inherited gene mutations raise the risk of melanoma?
Mutations in the following genes are passed down from parent to child and can increase the risk of melanoma:
CDKN2A gene
The CDKN2A gene is a regulator of cell division. Mutations in this gene are the most common cause of inherited melanoma.
The risk of melanoma in CDKN2A mutation carriers is approximately 14% by age 50 years, 24% by age 70 years and 28% by age 80 years.
Since many people who have mutations in CDKN2A will develop melanoma during their lifetime, commercial tests have been developed for CDKN2A abnormalities, although it is not clear if knowing the results of the test will benefit people carrying the gene.
How do CDKN2A gene mutations cause melanoma?
CDKN2A encodes two distinct proteins:
- INK4A (inhibitor of cyclin-dependent kinase 4; also known as p16INK4a)
- ARF (alternate open reading frame; also known as p14ARF in humans).
Mutations in CDKN2A can disrupt the functions of either or both of these proteins.
INK4A inhibits the G1 phase of the cell cycle which is mediated by the G1 cyclin-dependent kinases (CDKs) 4/6 which phosphorylate and inactivate the retinoblastoma protein (RB), thereby allowing entry into the S-phase of the cell cycle. The G1 cell cycle arrest phase must be bypassed before cell division.
Loss of INK4A function through mutation prevents the binding of INK4A to CDK4 and promotes RB activation through hyperphosphorylation resulting in unconstrained cell cycle progression.
ARF protein has been shown to promote the stabilisation of p53, a critical tumour suppressor and proapoptotic protein (a protein associated with programmed cell death) by preventing Mdm2 (murine double minute protein)-mediated degradation of p53.
Inactivation of ARF leads to degradation of the p53 protein and rite of passage to unrestrained cell multiplication.
Families with ARF mutations appear to develop cutaneous melanoma and neural system tumours, whereas, in families with INK4 mutations, cutaneous melanoma and pancreatic cancer often cluster in the same pedigree.
MDm2 gene
Mdm2 is an essential negative regulator of the p53 tumour suppressor protein. It is the name of a gene as well as the protein encoded by that gene.
Mutations in the MDm2 gene predispose women, but not men, to develop melanoma at a younger age (less than 50 years old). Having this mutation may be even more potent than other melanoma risk factors such as a history of blistering sunburns, fair skin, and freckling.
CDK4 gene
Other loci (position of a gene on a chromosome) that genetically interact with CDKN2A have also been implicated in melanoma risk.
A much smaller subset of melanoma-prone families harbours germline mutations in cyclin-dependent protein kinase 4 (Cdk4) – a protein kinase that is normally inhibited by INK4A.
When CDK4 is mutated, the enzyme is rendered resistant to INK4A and functions as a heritable autosomal dominant oncogene increasing melanoma risk.
RB1 gene
Germline mutations in the retinoblastoma gene (RB1) itself, in the INK4A–CDK4/6–RB pathway, could lead to a 4-fold to 20-fold increased risk of melanoma.
These findings indicate that multiple mechanisms that function to bypass the Rb-dependent G1 cell cycle arrest are involved in the development of malignant melanoma.
MC1R gene
Increasing evidence has shown that the greater the number of variations in a gene called MC1R (melanocortin-1 receptor), the greater the risk for inherited melanoma.
The MC1R gene plays an important role in determining if a person has red hair, fair skin, and sensitivity to UV radiation. People who have olive and darker skin and who carry one or more variations of this gene have a higher than average risk for melanoma.
Having the MC1R mutation carries a more moderate risk than the CDKN2A or CDK4 gene mutations.
How do MC1R gene mutations cause melanoma?
MC1R codes for a seven-transmembrane G protein-coupled receptor expressed on the surface of melanocytes. It is a receptor for α-melanocyte stimulating hormone (α-MSH) and its antagonist, agouti.
The binding of α-MSH to the cell membrane receptor on a melanocyte leads to a switch in melanin from the red/yellow phaeomelanin pigments to the brown/black eumelanin. The black eumelanin is photoprotective against the damaging effects of the sun’s ultraviolet (UV) radiation.
Mutations in MC1R can affect the change from phaeomelanin to eumelanin pigment and increase melanoma risk.
TYR, TYRP1, and ASIP genes
Recently, other genes involved with skin pigment have been identified that may also increase susceptibility to inherited melanoma.
These genes encode the following proteins: TYR (tyrosinase), TYRP1 (TYR related protein 1), and ASIP (agouti signalling protein).
Tyrosinase is a copper-containing enzyme that catalyses the production of melanin and other pigments and is encoded by the TYR gene.
TYRP1 is a protein involved in melanin production and is encoded by the TYRP1 gene.
Tyrp1 is involved in stabilising the tyrosinase enzyme and modulating its catalytic activity. Tyrp1 also affects melanocyte proliferation and melanocyte cell death.
Agouti signalling protein is a product of the Agouti gene, also called ASIP. ASIP affects the quality of hair pigmentation and act as an inhibitor of the α-melanocyte stimulating hormone.
Mutations in TYR, TYRP1 or ASIP genes can result in the production of abnormal proteins and increase melanoma risk.
Non-inherited gene mutations that increase the risk of melanoma
Mutations in the following genes are not inherited but are acquired in a given lifetime, for example, due to environmental factors such as exposure to UV rays of the sun. They can nevertheless increase the risk of melanoma.
BRAF gene
Studies have identified a non-inherited mutation in the BRAF gene that appears to be the most common event in the process that leads to melanoma; it has been observed in up to 66% of malignant melanomas, particularly those arising in younger patients.
Given the prevalence of BRAF mutations in melanoma, there has been intense interest in selective BRAF inhibitors for disease treatment.
Two drugs have been approved by the FDA for use in confirmed mutant-BRAF metastatic melanoma: vemurafenib and dabrafenib.
CDKN2A gene
Mutations in the CDKN2A gene have also been reported in non-inherited cases of melanoma.
EGF gene
Researchers are studying mutations in a gene that makes a substance called epidermal growth factor (EGF). EGF plays a role in skin cell growth and wound healing, and it may account for many non-inherited cases of melanoma.
Fas gene
Mutations in genes that regulate Fas proteins, which are involved in a natural process of cell self-destruction called apoptosis, can cause melanoma cells to proliferate out of control.
PTEN gene
Mutations in the inhibitory tumour suppressor gene PTEN (phosphatase and tensin homologue) occur in 30–50% of melanomas.
The PTEN protein has at least two biochemical functions: it has both lipid phosphatase and protein phosphatase activity.
The lipid phosphatase activity of PTEN arrests cell-cycle progression at G1/S. The protein phosphatase activity of PTEN is involved in cell spreading and migration.
The cell cycle, or cell-division cycle, is a series of events that take place in a cell leading to its division and duplication (replication). It consists of four distinct phases: G1 phase, (ensures that everything is ready for DNA synthesis), S phase (DNA replication occurs during this phase), G2 phase (collectively known as interphase ensures that everything is ready to enter the M phase) and M phase (mitosis; i.e. cell duplication).
The combined effects of the loss of PTEN lipid and protein phosphatase activity may result in abnormal cell growth as well as abnormal cell spreading and migration.
Role of genetic testing to determine melanoma risk
There are no widely accepted guidelines for managing families with an increased hereditary risk for developing cutaneous melanoma.
The American Society of Clinical Oncology has stated that screening genetic tests for CDKN2A and CDK4 have not yet been shown to be of medical benefit.
GenoMEL (the Melanoma Genetics Consortium) has published consensus statements recommending against routine genetic testing for familial cutaneous melanoma except in rare circumstances.
The rationale for their recommendations is as follows:
- mutations have not yet been detected in the majority of families with hereditary cutaneous melanoma
- our understanding of melanoma risk (penetrance) to carriers of gene mutations is limited
- other factors (i.e. environment) appear to influence the risk
- negative testing may provide false reassurance.
GenoMEL recommends that:
- all high-risk family members are trained in skin self-examination
- have baseline skin examinations at age ten years by a physician specialist
- continue clinical skin examinations every six months by a physician specialist until melanocytic naevi are stable
- have annual or biannual examinations after that time depending on numbers of atypical naevi regardless of CDKN2A mutation status.
The lack of definitive evidence showing clinical benefit from genetic testing further supports continued strict surveillance and optimised solar protection as primary goals for prevention of malignant melanoma.
References
- Hocker TL, Singh MK, Tsao H. "Melanoma genetics and therapeutic approaches in the 21st century: moving from the benchside to the bedside." Invest Dermatol. 2008; 128(11):2575-95.
- Lin J, Hocker TL, Singh M, Tsao H. "Genetics of Melanoma Predisposition." Br J Dermatol. 2008; 159(2):286-9.
- Firoz EF, Warycha M, Zakrzewski J, et al. Association of MDM2 SNP309, age of onset, and gender in cutaneous melanoma. Clin Cancer Res. 2009; 15(7):2573-80.
- Kefford RF, Newton Bishop JA, Bergman W, Tucker MA. Counseling and DNA testing for individuals perceived to be genetically predisposed to melanoma: a consensus statement of the Melanoma Genetics Consortium. J Clin Oncol. 1999; 17:3245-51.
- Kefford R, Bishop JN, Tucker M et al. Genetic testing for melanoma. Lancet Oncol 2002; 3:653-4.
- Flaherty KP, Sosman J, Jim K, et al. Phase I study of PLX4032: proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J Clin Oncol 2009; 27: 9000.
On DermNet
- Genes and melanoma – the simple version
- Melanocortin-1 receptor gene
- Blood-based melanoma detection tests
- Genetic testing for melanoma
- Interferon regulatory factor 4 gene
- Melanoma
- Atypical naevi
- Sun protection
- Skin self examination
- Melanoma in skin of colour
Other websites
- GenoMEL – the melanoma genetics consortium
- Clinical guidelines for the management of melanoma in Australia and New Zealand, eBook
- Guidelines to the management of cutaneous melanoma – British Association of Dermatologists
- BRAF targeted therapy in melanoma – Medscape News
- Patient Fact Sheet: BRAF Testing – Pathlab
