Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Blood-based melanoma detection — extra information
Lesions (cancerous) Diagnosis and testing
Blood-based melanoma detection
Author(s): Pauline Zaenker, Leslie Calapre, Michael Clark, Gabriela Marsavela, Carlos Aya-Bonilla, Dr Elin Gray, and Prof Mel Ziman, the School of Medical and Health Sciences, Edith Cowan University, Joondalup, Perth, WA, Australia. Michael Clark and Prof Mel Ziman are also part of the School of Biomedical Science, The University of Western Australia, Crawley, WA, Australia. DermNet Editor in Chief: Adjunct A/Professor Amanda Oakley. Copy edited by Gus Mitchell. July 2018.
Introduction - melanoma detection Introduction Introduction - cancer-derived blood-based biomarkers Progress Blood-based detection of other cancers Tests Benefits Disadvantages Side effects and risks
How is melanoma usually detected?
Cutaneous melanoma is generally first suspected during a skin check and the diagnosis is confirmed by a positive skin biopsy. Over the last few decades, techniques such as dermoscopy and total body photography have improved melanoma diagnosis and surveillance. However, melanoma detection remains challenging [1].
At later stages of the cancer, PET or CT scans may be used to follow disease progression.
What is blood-based melanoma detection?
Blood-based melanoma detection is the detection of melanoma-derived components in the patient’s blood. Blood-based melanoma detection may prove particularly useful for diagnosis, prognosis or surveillance where the primary melanoma is inaccessible or where there are numerous secondary growths (metastases).
Blood-based cancer detection is also known as liquid biopsy.
What are cancer-derived blood-based biomarkers?
Blood-based biomarkers related to cancer include:
- Tumour-associated autoantibodies (TAAbs)
- Circulating tumour cells (CTCs)
- Circulating tumour DNA (ctDNA)
- Tumour-derived exosomes (vesicles that carry cell products outside the cell) (TEXs).
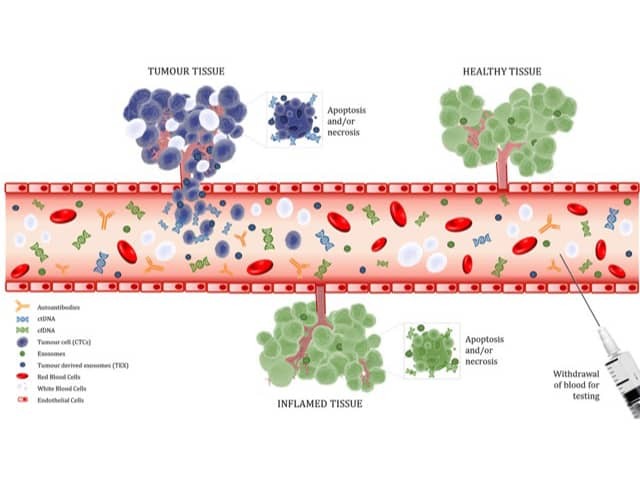
Biomarkers in bloodstream
Figure 1. Tumour biomarkers released into the peripheral bloodstream. Cell-free DNA (cfDNA) and exosomes are released by non-neoplastic tissue while circulating tumour cells, tumour-derived exosomes and tumour-derived exosomes are released by tumours. White blood cells that infiltrate the tumour enable the production of autoantibodies that target the tumour tissue. These markers, along with red blood cells and white blood cells, are found in the blood. Image created by Gabriela Marsavela using resources obtained from somersault18:24.
What is the place of blood-based melanoma detection?
Blood-based melanoma detection is experimental and currently not in clinical use.
Research into this field is advancing worldwide, and several blood-based melanoma detection and surveillance tests are expected to become available to clinics in the next few years.
Blood-based melanoma detection tests are expected to serve as complementary to current practices, such as skin checks, radiological imaging and pathological tumour tissue testing for melanoma, until the validity and reliability of the new tests are established in large numbers of patients. At this stage, blood-based melanoma detection will not influence treatment until the benefit of the tests are proven and validated.
Blood-based detection of other cancers
A small number of tests are used for the diagnosis and monitoring of other cancers.
- The prostate specific antigen (PSA) test is commonly used for prostate cancer screening [2].
- Tumour associated autoantibody-based diagnostic blood tests have been developed, including the EarlyCDT®-Lung test for lung cancer detection and 'PAULA’s test' for non-small cell lung cancer (NSCLC); however, these are not yet in routine use [3,4].
- The US Food and Drug Administration (FDA) has approved the use of a circulating tumour cell detection and enumeration kit (CellSearch®) for cancer prognostication in breast, prostate and colorectal cancer, and this is increasingly used in clinical trials of new therapies [5].
- ctDNA can be analysed to detect the tyrosine kinase-resistant EGFR p.T790M mutation in patients with NSCLC, as these mutations cause resistance to targeted tyrosine kinase inhibitor therapies, and their early detection will allow patients to be switched to second-line or alternate drugs [6].
What tests are involved in blood-based melanoma detection?
Depending on the suspected stage of the melanoma, different tests may be used to detect a particular biomarker in the blood (figure 1).
Tumour-associated autoantibodies
Autoantibodies are antibodies produced by the patient’s own immune system in response to antigens (proteins) that normally reside within the body.
- Exactly why this potentially self-destructive immune response occurs in melanoma is not yet fully understood, but may be a result of the release of intracellular antigens into the circulation from cells that turned into cancer cells and then died [7].
- These antigens are present in normal cells, where they are usually hidden from immune cells.
- They become detectable in the blood of cancer patients, and autoantibodies are produced in response.
- TAAbs are thought to be the first biomarker for melanoma in the blood [8].
Using a large cohort of 104 patients with early-stage melanoma and 105 healthy volunteers, the melanoma research team at Edith Cowan University in Perth, Australia, has recently identified 139 autoantibodies with the potential to aid in primary melanoma diagnosis. The team are currently developing a blood-based melanoma detection test that uses a combination of 10 of these autoantibodies that may detect the cancer with 81.5% overall accuracy.
Circulating tumour cells
CTCs are single tumour cells that shed into the bloodstream, with the potential to seed new tumours (metastases) in distant tissues and organs, which is the main cause of death in cancer patients [9,10].
- CTCs are associated with poor prognosis and failure of treatment [11,12].
- The absence or a decrease in the number of CTCs may indicate a favourable response to treatment.
- Microfluidic chips (microscopic polymer or glass chips etched with micro-channels) are being used to isolate CTCs, based on their larger size compared with other cells [13].
- Multiple CTC subtypes have been found in the blood of patients with melanoma; these CTC subtypes characterised by the different proteins on their cell surfaces and respond differently to treatment [14].
Many patients develop resistance to melanoma therapies and new drugs are constantly being trialled. Human CTCs may be used to test the efficacy of drugs either in vitro or in mice, as exemplified in a recent study from Cancer Research UK [15].
Circulating tumour DNA
Plasma-derived ctDNA are short DNA fragments that are released into the patient's circulation by dying tumour cells.
- The detection of plasma ctDNA provides a reliable marker of overall disease and can be used for routine monitoring in a patient with metastatic (stage IV) melanoma [15–23].
- Plasma ctDNA detects all the patient-specific melanoma-specific mutations in the patient at any one time.
- Low or undetectable ctDNA levels prior to treatment are associated with improved patient response and may have prognostic value [16–20].
- The concentration of ctDNA in the patient's blood correlates with tumour burden and can provide accurate information about response to therapy [15, 16]. A decline in ctDNA levels during treatment is indicative of response, while rebounding levels suggest disease progression and/or resistance to therapy [15, 16, 22].
Clinical trials are required to define the benefits of ctDNA-guided clinical decisions before plasma ctDNA can be used routinely in the management of melanoma.
Tumour-derived exosomes
Exosomes are small vesicles 50–100 nm in diameter produced by all cells in the body. TEXs are currently being researched as potential biomarkers in multiple cancers [24–27]. Current work in melanoma exosomes is limited.
- Initial work has shown that specific micro-RNAs and proteins within exosomes are suitable sensitive biomarkers of disease.
- Exosome-associated serum tumour markers include S100 and melanoma inhibitory activity (MIA), which are used to detect early-stage melanoma [28].
- Levels of tyrosinase-related protein 2 (TRP-2) isolated from exosomes were reduced in patients with late stage (stage 4) melanoma when compared to healthy controls.
- Levels of microRNA-125b were able to differentiate a particular set of patients with late-stage melanoma from healthy controls [28,29].
- More recent research has shown that exosomes from patients with melanoma can predict patient response to immunotherapy [30].
What are the benefits of blood-based melanoma detection?
Blood collection and processing are routine procedures performed by most hospitals and pathology laboratories.
- Blood-based melanoma detection is less invasive than the surgical excision of a tumour.
- The tests can be performed regularly to monitor for any sign of disease (in high-risk patients) and to detect disease progression or response to treatment.
- They are expected to be time efficient and relatively inexpensive.
Any insights gathered from the research into blood-based melanoma detection could inform the development of similar tests for other cancers.
- Autoantibody tests may offer the opportunity to detect melanoma in its early stages, possibly preceding the clinical manifestation of the tumour by several months to years [31, 32]. TAAbs persist at stable concentrations in blood for extended periods of time [33].
- Detecting specific melanoma CTC subtypes may help determine prognosis.
- The presence of ctDNA may be used for the surveillance of metastatic melanoma in patients undergoing systemic treatment.
- TEXs have the potential to provide a comprehensive, real-time investigation of melanoma protein, DNA and RNA.
What are the disadvantages of blood-based melanoma detection?
Complex, time-consuming and expensive research is needed before blood-based melanoma detection can be used in routine clinical practice.
- CTCs are rare (1–10 cells per 10 mL of blood) so a large volume of blood may need to be collected on a regular basis if the test is to be used to monitor cancer progression and response to treatment.
- CTCs are fragile, so fresh blood samples are required to isolate and analyse these cells.
- ctDNA has a limited efficacy in patients with brain metastases and in those with low disease burden [34, 35].
What are the side effects and risks of blood-based melanoma detection?
The side effects and risks of blood-based melanoma detection may include:
- Slight discomfort, due to the needle prick when the blood is collected
- A need to monitor for potential iron deficiency anaemia in people undergoing regular, large blood collections
- Possible false readings, as the tests may not be 100% accurate.
References
- Loescher LJ, Janda M, Soyer HP, et al. Advances in skin cancer early detection and diagnosis. Semin Oncol Nurs 2013; 29: 170–81. DOI: 10.1016/j.soncn.2013.06.003. PubMed
- Prostate Cancer Foundation of Australia. Clinical practice guidelines on PSA testing. 2018. Available at: http://www.prostate.org.au/awareness/for-healthcare-professionals/clinical-practice-guidelines-on-psa-testing/
- Chapman CJ, Healy GF, Murray A, et al. EarlyCDT®-Lung test: improved clinical utility through additional autoantibody assays. Tumour Biol 2012; 33: 1319–26. DOI: 10.1007/s13277-012-0379-2. PubMed
- Doseeva V, Colpitts T, Gao G, Woodcock J, Knezevic V. Performance of a multiplexed dual analyte assay for the early detection of non-small cell lung cancer. J Transl Med 2015; 13: 55. DOI: 10.1186/s12967-015-0419-y. PubMed
- Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol 2010: 2010; 617421. DOI: 10.1155/2010/617421. PubMed
- Kwapisz D. The first liquid biopsy test approved. Is it a new era of mutation testing for non-small cell lung cancer? Ann Transl Med 2017; 5(3): 46. DOI: 10.21037/atm.2017.01.32. PubMed
- Zaenker P, Gray ES, Ziman MR. Autoantibody production in cancer — the humoral immune response toward autologous antigens in cancer patients. Autoimun Rev 2016; 15: 477–83. DOI: 10.1016/j.autrev.2016.01.017. PubMed
- Kaae J, Wohlfahrt J, Boyd HA, Wulf HC, Biggar RJ, Melbye M. The impact of autoimmune diseases on the incidence and prognosis of cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev 2007; 16: 1840–4. DOI: 10.1158/1055-9965.EPI-07-0459. PubMed
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009; 9: 274–84. DOI: 10.1038/nrc2622. PubMed
- Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 2011; 147: 992–1009. DOI: 10.1016/j.cell.2011.11.016. PubMed
- Klinac D, Gray E, Freeman J, et al. Monitoring changes in circulating tumour cells as a prognostic indicator of overall survival and treatment response in patients with metastatic melanoma. BMC Cancer 2014; 14: 423. DOI: 10.1186/1471-2407-14-423. Journal
- Khoja L, Lorigan P, Zhou C, et al. Biomarker utility of circulating tumor cells in metastatic cutaneous melanoma. J Invest Dermatol 2013; 133: 1582–90. DOI: 10.1038/jid.2012.468. PubMed
- Aya-Bonilla CA, Marsavela G, Freeman JB, et al. Isolation and detection of circulating tumour cells from metastatic melanoma patients using a slanted spiral microfluidic device. Oncotarget 2017; 8: 67335–68. DOI: 10.18632/oncotarget.18641. PubMed
- Gray ES, Reid AL, Bowyer S, et al. Circulating melanoma cell subpopulations: their heterogeneity and differential responses to treatment. J Invest Dermatol 2015; 135: 2040–8. DOI: 10.1038/jid.2015.127. PubMed
- Girotti MR, Gremel G, Lee R, et al. Application of sequencing, liquid biopsies, and patient-derived xenografts for personalized medicine in melanoma. Cancer Discov 2016; 6: 286–99. DOI: 10.1158/2159-8290.CD-15-1336. PubMed
- Gray ES, Rizos H, Reid AL, et al. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget 2015; 6: 42008–18. DOI: 10.18632/oncotarget.5788. PubMed
- Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013; 31: 3205–11. DOI: 10.1200/JCO.2013.49.8691. PubMed
- Sanmamed MF, Fernandez-Landazuri S, Rodriguez C, et al. Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin Chem 2015; 61: 297–304. DOI: 10.1373/clinchem.2014.230235. PubMed
- Santiago-Walker A, Gagnon R, Mazumdar J, et al. Correlation of BRAF mutation status in circulating-free DNA and tumor and association with clinical outcome across four BRAFi and MEKi clinical trials. Clin Cancer Res 2016; 22: 567–74. DOI: 10.1158/1078-0432.CCR-15-0321. PubMed
- Gonzalez-Cao M, Mayo-de-Las-Casas C, Molina-Vila MA, et al. BRAF mutation analysis in circulating free tumor DNA of melanoma patients treated with BRAF inhibitors. Melanoma Res 2015; 25: 486–95. DOI: 10.1097/CMR.0000000000000187. PubMed
- Schreuer M, Meersseman G, Van Den Herrewegen S, et al. Quantitative assessment of BRAF V600 mutant circulating cell-free tumor DNA as a tool for therapeutic monitoring in metastatic melanoma patients treated with BRAF/MEK inhibitors. J Transl Med 2016; 14: 95. DOI: 10.1186/s12967-016-852-6. PubMed
- Wong SQ, Raleigh JM, Callahan J, et al. Circulating tumor DNA analysis and functional imaging provide complementary approaches for comprehensive disease monitoring in metastatic melanoma. JCO Precis Oncol 2017; April 27. Epub. DOI: 10.1200/PO.16.00009. Journal
- Lee JH, Long GV, Boyd S, et al. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann Oncol 2017; 28: 1130–6. DOI: 10.1093/annonc/mdx026. PubMed
- Hannafon BN, Trigoso YD, Calloway CL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res 2016; 18(1): 90. DOI: 10.1186/s13058-016-0753-x. PubMed Central
- Ogata-Kawata H, Izumiya M, Kurioka D, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS ONE 2014; 9: e92921. Journal
- Jakobsen KR, Paulsen BS, Bæk R, Varming K, Sorensen BS, Jørgensen MM. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles 2015; 4: 26659. DOI: 10.3402/jev.v4.26659. PubMed
- Khan S, Jutzy JMS, Valenzuela MMA, et al. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS ONE 2012; 7: e46737. DOI: 10.1371/journal.pone.0046737. Journal
- Alegre E, Zubiri L, Perez-Gracia JL, et al. Circulating melanoma exosomes as diagnostic and prognosis biomarkers. Clin Chim Acta 2016; 454: 28–32. DOI: 10.1016/j.cca.2015.12.031. PubMed
- Alegre E, Sanmamed MF, Rodriguez C, Carranza O, Martín-Algarra S, González A, et al. Study of circulating microRNA-125b levels in serum exosomes in advanced melanoma. Arch Pathol Lab Med 2014; 138: 828–32. DOI: 10.5858/arpa.2013-0134-OA. PubMed
- Tucci M, Passarelli A, Mannavola F, et al. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma. OncoImmunology 2018; 7(2): e1387706. DOI: 10.1080/2162402X.2017.1387706. PubMed
- Zayakin P, Ancāns G, Silina K, et al. Tumor-associated autoantibody signature for the early detection of gastric cancer. Int J Cancer 2013; 132: 137–47. DOI: 10.1002/ijc.27667. PubMed
- Caron M, Choquet-Kastylevsky G, Joubert-Caron R. Cancer immunomics: using autoantibody signatures for biomarker discovery. Mol Cell Proteomics 2007; 6: 1115–22. PubMed
- Anderson KS, Cramer DW, Sabani S, et al. Autoantibody signature for the serologic detection of ovarian cancer. J Proteome Res 2015; 14: 578–86. DOI: 10.1021/pr500908n. PubMed
- De Mattos-Arruda L, Mayor R, Ng CKY, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun 2015; 6: 8839. DOI: 10.1038/ncomms9839. Journal
- Momtaz P, Pentsova E, Abdel-Wahab O, et al. Quantification of tumor-derived cell free DNA (cfDNA) by digital PCR (DigPCR) in cerebrospinal fluid of patients with BRAFV600 mutated malignancies. Oncotarget 2016; 7: 85430–36. DOI: 10.18632/oncotarget.13397. PubMed Central
On DermNet
Other websites
- ECU Melanoma Research Group — Edith Cowan University School of Medical and Health Sciences
