Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Systemic corticosteroid — extra information
Systemic corticosteroid
Author: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, 1997. Updated February 2016.
Introduction
Uses
How it works
Differences
Dosage
Side effects and risks - short-term systemic steroids
Side effects and risks - long-term systemic steroids
Risks
Effects of dose reduction
Monitoring
What is a systemic corticosteroid?
A corticosteroid taken by mouth or given by intramuscular injection is often called a systemic steroid. Systemic steroids are synthetic derivatives of the natural steroid, cortisol, produced by the adrenal glands, and have profound anti-inflammatory effects.
Systemic (cortico)steroids are also called glucocorticoids or cortisones. They include:
- Prednisone
- Prednisolone
- Methylprednisolone
- Beclomethasone
- Betamethasone
- Dexamethasone
- Hydrocortisone
- Triamcinolone.
Prednisone and prednisolone are equivalent and are the most commonly prescribed oral corticosteroids for inflammatory skin diseases. Oral prednisone is the most commonly prescribed systemic steroid in New Zealand.
Fludrocortisone is predominantly a mineralocorticoid and its anti-inflammatory effects are minimal.
What is prednisone used for in dermatology?
Prednisone is used for a few days (short-term) to indefinitely (long-term) in a wide variety of skin conditions including:
Systemic steroids are best avoided in patients with psoriasis.
How does a systemic steroid work?
Systemic steroids work in the same way as natural cortisol. Natural cortisol has important effects on the body, including regulation of:
- Protein, carbohydrate, lipid and nucleic acid metabolism
- Inflammation and immune response
- Distribution and excretion of water and solutes
- Secretion of adrenocorticotrophic hormone (ACTH) from the pituitary gland.
How do systemic steroids differ?
Systemic steroids differ in dose, mineralocorticoid potency, half-life (duration of action) and how effectively they suppress the hypothalamic-pituitary-adrenal (HPA) axis (suppression leads to reduced production of natural cortisol).
Comparison of systemic steroids*
Drug |
Cortisone |
Hydrocortisone |
Prednisone |
Methylprednisolone |
Dexamethasone |
|---|---|---|---|---|---|
Equivalent dose |
25 |
20 |
5 |
4 |
0.75 |
Mineralocorticoid potency |
2+ |
2+ |
1+ |
0-0.5+ |
0 |
Biological half-life |
8–12 |
8–12 |
24–36 |
24–36 |
36–54 |
Daily dose causing HPA axis suppression (mg) |
25–30 |
20–30 |
7.5 |
7.5 |
1–1.5 |
* Comparison of systemic corticosteroids – Vancouver Coastal Health Formulary tool. Accessed 12 July 2014.
What is the usual dose of prednisone?
Generally, a higher dose of prednisone, such as 40–60 mg daily, is prescribed at first, to gain control of the skin condition. In 2–4 weeks, the dose is reduced.
Prednisone is best taken as a single dose in the morning, which is thought to reduce steroid-induced suppression of the pituitary-adrenal axis compared to evening dosing.
The maintenance dose should be kept as low as possible to minimise adverse effects.
Steroid dose is commonly characterised as:
- Low dose, eg < 10mg/day of prednisone
- Medium dose, eg 10–20 mg/day of prednisone
- High dose, eg > 20mg/day of prednisone, sometimes more than 100 mg/day
Treatment for less than one month is considered short-term treatment. Corticosteroids for a few days or weeks are relatively safe when prescribed for acute dermatitis. Treatment continuing for more than 3 months is regarded as long-term, and results in the majority of undesirable side effects.
What are the side effects and risks of short-term systemic steroid?
Side effects are rarely serious if a systemic steroid has been prescribed for one month or less. The following problems may arise, particularly when higher doses are taken:
- Sleep disturbance
- Increased appetite
- Weight gain
- Increase in postprandial blood sugar
- Psychological effects, including increased or decreased energy.
Rare and potentially serious side effects of a short course of corticosteroid include:
- Severe infection
- Mania, psychosis, delirium, depression with suicidal intent
- Heart failure
- Peptic ulceration
- Diabetes mellitus
- Avascular necrosis of the hip.
The risk of a serious side effect increases with increasing dose.
See DermNet's page on prophylactic treatments for dermatology patients on systemic corticosteroid.
What are the side effects and risks of long-term systemic steroid?
Nearly everyone on a systemic steroid for more than a month suffers from some adverse effects, depending on daily dose and how long they have been on the drug. The main concerns are infections, hypertension, diabetes, osteoporosis, avascular necrosis, myopathy, cataracts, and glaucoma. The list that follows is incomplete.
Cutaneous adverse effects
Cutaneous adverse effects from long-term systemic steroids may include:
- Bacterial infections: cellulitis, wound infection
- Fungal infections: tinea, candida, pityriasis versicolor
- Viral infections: herpes zoster
- Skin thinning, purpura, fragility, telangiectasia and slow wound healing, especially in sun-damaged areas
- Stretch marks (striae) under the arms and in the groin
- Steroid acne
- Hypertrichosis and hair loss.
Adverse effects of systemic steroids
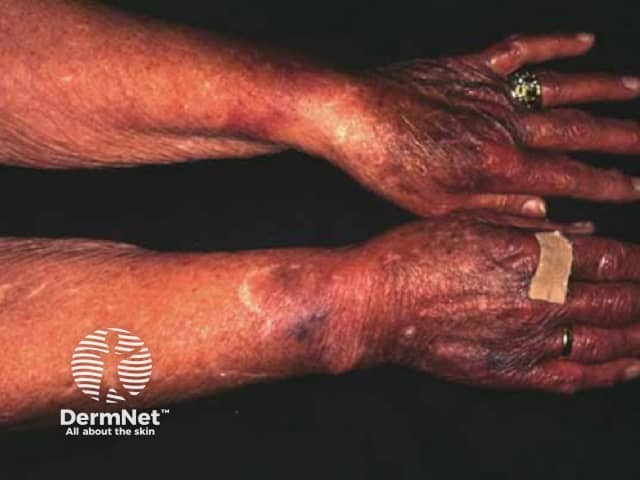
Easy bruising

Moon face
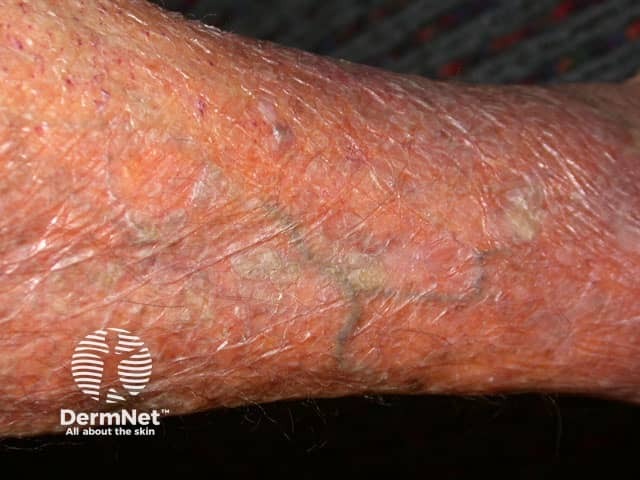
Skin thinning
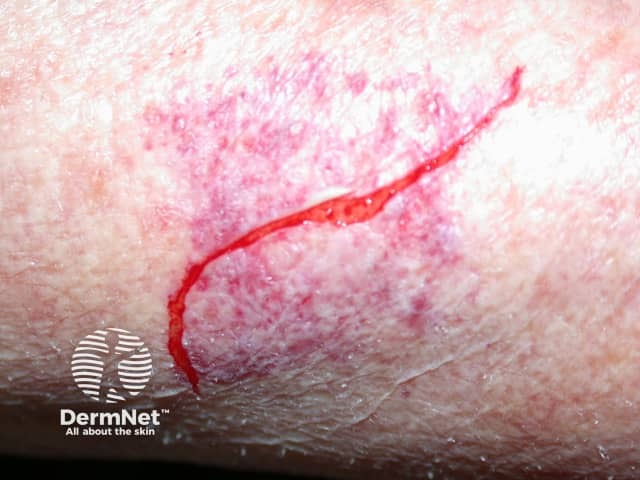
Fragile skin
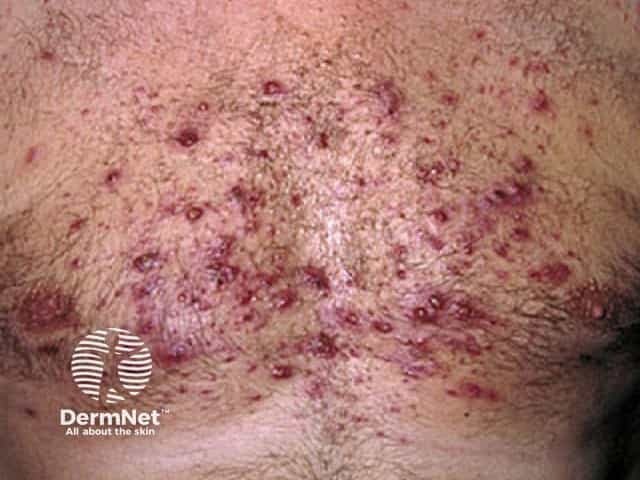
Acne
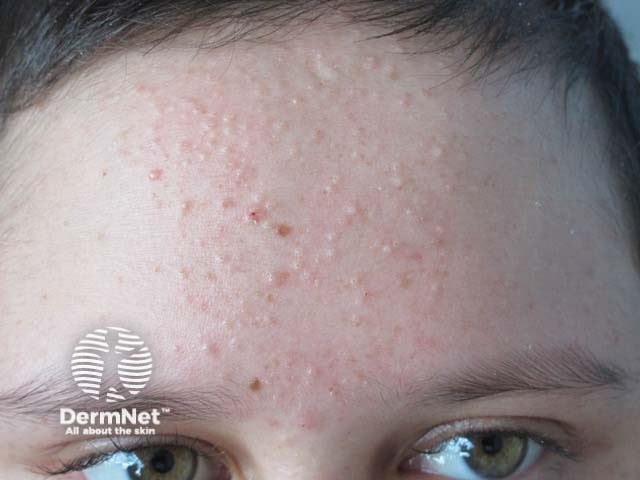
Acne due to systemic steroids
Effects on body fat
- Redistribution of body fat: moon face, buffalo hump, truncal obesity
- Weight gain: increased appetite and food intake
Effects on the eye
- Glaucoma
- Posterior subcapsular cataracts; children are more susceptible than adults
- Eyelid oedema and exophthalmos
- Central serous chorioretinopathy
Vascular disease
- Hypertension
- Ischaemic heart disease
- Stroke and transient ischaemic attack (TIA)
The effects of systemic steroids on atherosclerotic vascular disease may be due to complex metabolic changes, including:
- Hyperlipidaemia
- Peripheral insulin resistance and hyperinsulinaemia.
Gastrointestinal tract
- Dyspepsia, gastritis, peptic ulceration and perforation of the gut, especially in patients also taking non-steroidal anti-inflammatory drugs
- Acute pancreatitis
- Fatty liver
- Fluid balance
- Sodium and fluid retention cause leg swelling and weight increase
- Potassium loss causes general weakness
Reproductive system
- Irregular menstruation
- Hirsutism
- Lowered fertility in men (hypogonadism) and women
- Possible fetal growth retardation in women taking prolonged courses of steroids during pregnancy
- Breastfeeding can usually continue but infant should be monitored for adrenal suppression if the mother on > 40 mg prednisone daily
Musculoskeletal system
- Bone fracture
- Osteoporosis
- Osteonecrosis, especially hip
- Myopathy affecting shoulders and thighs
- Tendon rupture
- Growth restriction in children
Osteoporosis is particularly common in smokers, postmenopausal women, the elderly, underweight or immobile, and patients with diabetes or lung problems. Osteoporosis may result in fractures of the spine, ribs or hip joint with minimal trauma. These occur after the first year in 10–20% of patients treated with more than 7.5 mg prednisone daily. It is estimated that up to 50% of patients on long-term prednisone will develop bone fractures. Vertebral fractures are more common in patients on steroids, even in those with normal bone density.
Nervous system
- Psychological effects: mood changes, increased energy, excitement, euphoria, agitation
- Less often: hypomania, psychosis, delirium, memory loss, depression, anxiety, personality change
- Insomnia and sleep disturbance
- Shakiness and tremor
- Headaches
Metabolic effects
- Transient or persistent diabetes in previously non-diabetic patients
- Higher blood sugar levels in patients with diabetes mellitus
- Cushing syndrome
Immune response
- Raised neutrophil and total white cell count are usual on prednisone
- Impaired innate and acquired immunity
- Increased susceptibility to tuberculosis
- Increased severity of measles, varicella
- Reduced efficacy and increased risk of vaccines
Live vaccines such as polio or MMR (measles, mumps, rubella) should not be given to patients taking ≥ 20 mg prednisone daily. It is safe and advisable to have other routine immunisations, such as annual influenza vaccination.
Risks during intercurrent illness or surgery
Significant intercurrent illness, trauma, or surgical procedure requires a temporary increase in corticosteroid dose, or if already stopped, a temporary re-introduction of corticosteroid treatment for up to twelve months after the steroids are stopped.
Patients who have taken ≥10 mg prednisone daily within 3 months of surgery requiring a general anaesthetic are advised to tell their anaesthetist so that intraoperative intravenous hydrocortisone can be added.
Effects of reducing the dose of systemic steroid
No tapering is necessary if a course of prednisone has been for less than one to two weeks. Steroid should be withdrawn slowly after longer courses, to avoid acute adrenal insufficiency, particularly if the medication has been taken for several months or longer.
Side effects from reducing prednisone may include:
- Fever
- Hypotension
- Tiredness
- Headaches
- Muscle and joint aches
- Weight loss
- Depression
- Rhinitis
- Conjunctivitis
- Painful itchy skin nodules.
Hypopituitary-pituitary-adrenal (HPA) axis suppression can persist for months or years after steroids are stopped.
Monitoring during steroid treatment
Regular monitoring during treatment with systemic steroid may include:
- Blood pressure
- Body weight
- Blood sugar
Patients on prednisone should be advised to avoid non-steroidal anti-inflammatory drugs and licorice.
Prevention of osteoporosis
Bone density scans should be considered for patients that have taken or are expected to take 7.5 mg or more of prednisone each day for three months or longer. Baseline fracture risk can be estimated from T-scores.
- See DermNet's page on prophylactic treatments for dermatology patients on systemic corticosteroid
Current recommendations are:
- Bisphosphonate therapy (alendronate, etidronate, zoledronic acid) for individuals with femoral T-scores <-2.5. It reduces fracture risk by half.
- Smoking cessation
- A balanced diet, aiming for healthy body weight
- Minimal alcohol
- Regular weight-bearing exercise
- Consider the risk of falling and its mitigation
Calcium, vitamin D and oestrogen are no longer recommended for prophylaxis of osteoporosis, as adverse events outweigh the benefit.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Glucocorticoid therapy – New Zealand Formulary
- Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015 Jul;11(7):418-28. doi: 10.1038/nrendo.2015.71. Epub 2015 May 12. Review. PubMed PMID: 25963272.
- The Risk of Hyperglycaemia with Systemic Glucocorticoids. Prescriber Update 39(1): 3. Medsafe. March 2018
On DermNet
- Prophylactic treatments for dermatology patients on systemic corticosteroid
- Cushing syndrome
- Topical steroids
- Intralesional steroids
- Allergies explained
- Occupational skin disorders in homemakers
- Ramsay Hunt syndrome
Other websites
- FRAX® WHO Fracture Risk Assessment Tool
- Consumer medicine information and data sheets — Medsafe
- MyMedicines — New Zealand Formulary
- Drugs, Herbs and Supplements — MedlinePlus
- Medsafe on osteoporosis due to systemic steroids
- Oral treatment with corticosteroids — British Association of Dermatologists
- Prednisone — New Zealand Formulary Patient Information
