Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Tranexamic acid — extra information
Tranexamic acid
Author: Dr Faisal R Ali, Consultant Dermatologist, Dermatological Surgery and Laser Unit, St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom. DermNet Editor in Chief: Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell/Maria McGivern. August 2018.
Introduction
Uses
How it works
Contraindications
Dosage
Benefits
Disadvantages
Side effects and risks
Topical tranexamic acid
What is tranexamic acid?
Tranexamic acid is an antifibrinolytic agent and is commonly used for heavy menstrual bleeding.
When is tranexamic acid used in dermatology?
Tranexamic acid has been used (off-label) in dermatology for the improvement of melasma and as a maintenance treatment of hereditary angioedema and urticaria.
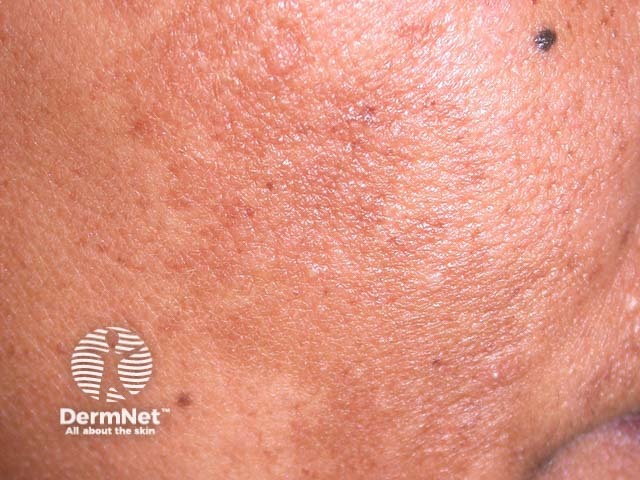
Melasma
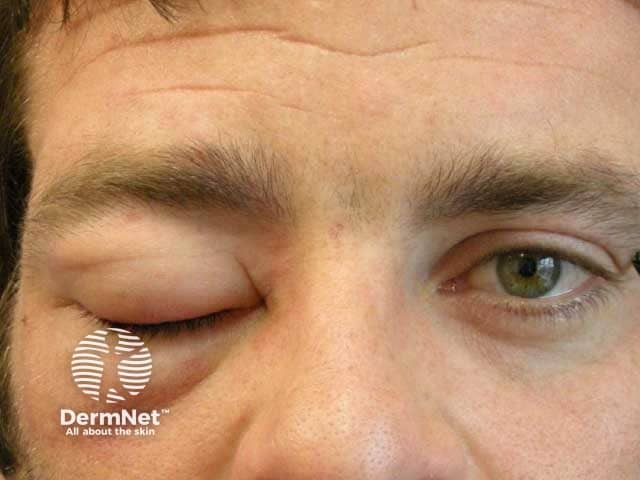
Angioedema
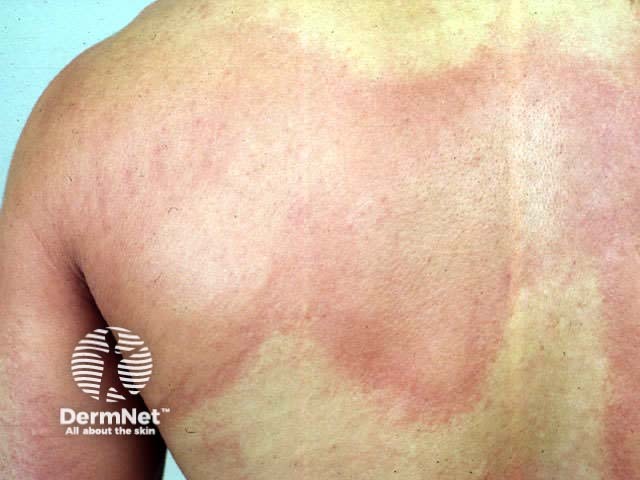
Urticaria
How does tranexamic acid work?
The mechanism of action of tranexamic acid in dermatological disorders is not fully understood. In melasma, the hypothesised mechanism of action of tranexamic acid includes the shrinkage of dermal vasculature and reduced melanin synthesis by altering the interaction of keratinocytes and melanocytes and reducing tyrosinase activity. In hereditary angioedema, tranexamic acid is thought to inhibit the bradykinin pathway.
What are the contraindications to tranexamic acid?
Contraindications to the use of tranexamic acid include:
- Personal or family history of coagulopathy
- Venous thromboembolism
- Ischaemic heart disease
- Stroke
- Hypersensitivity to tranexamic acid or any of its constituents
- Fibrinolytic conditions (the breakdown of fibrin that allows for clotting) due to consumption coagulopathy
- Severe renal insufficiency
- History of convulsions.
Tranexamic acid should not be given to patients with acquired disturbances of colour vision.
What dose of tranexamic acid is used in dermatology?
The dose used for melasma (250 mg twice daily) is comparable to the cumulative monthly dose used for menorrhagia (heavy menstruation). The dose suggested for hereditary angioedema is 1–1.5 g orally 2–3 times daily, as intermittent or continuous treatment, as determined by symptoms. A dose adjustment is needed in patients with severe renal failure.
What are the benefits of tranexamic acid?
Tranexamic acid is mostly well tolerated, with a long-standing safety profile, and it is not expensive.
When used in melasma, the reported success rate is up to 89%, with results appearing as early as eight weeks. When used in hereditary angioedema, 73% of patients reported a reduced frequency of attacks.
What are the disadvantages of tranexamic acid?
Tranexamic acid is not licensed for use in dermatological conditions. The safety profile for long-term use in melasma (particularly in older patients) remains largely unknown. On stopping treatment with tranexamic acid, the disease may recur.
What are the side effects and risks of tranexamic acid?
Tranexamic acid is mostly well-tolerated.
In the most extensive case series of patients treated with tranexamic acid for melasma for a median duration of 4 months (n=561), 7% of patients reported adverse events, including:
- Abdominal pain, bloating, nausea, and vomiting
- Numbness or itching of the face, lip, fingers, or toes
- Tinnitus
- Headache
- Transient amnesia
- Tremors
- Hypomenorrhoea (excessive menstrual bleeding) or dysmenorrhoea (painful menstruation)
- Increased hair shedding
- Facial hypertrichosis
- Lip or periorbital swelling
- Palpitations.
Of note, one patient suffered from a deep vein thrombosis but did have underlying protein S deficiency, which increases the risk of this complication. In a case series of patients using tranexamic acid for more than six months for maintenance therapy of hereditary angioedema (n=37), no thromboembolic events were reported.
While clinical evidence suggests no significantly increased risk of thrombosis in patients taking tranexamic acid, the possible risk of venous and arterial thrombotic complications cannot be entirely excluded. Before the use of tranexamic acid, the patient's risk factors for a thromboembolic disease should be considered and investigated.
Topical tranexamic acid
Topical tranexamic acid 5% cream is under investigation in the treatment of melasma and is showing promising results in the treatment of epidermal-type melasma. Randomised controlled trial data has not yet been reported (2019).
Intradermal injections of tranexamic acid have also been proposed as a treatment for localised melasma.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective analysis. J Am Acad Dermatol 2016; 75: 385–92. DOI: 10.1016/j.jaad.2016.03.001. Journal
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther 2017; 30(3): e12465. DOI: 10.1111/dth.12465. Journal
- Winterberger C, Boccon-Gibod I, Launay D, et al. Tranexamic acid as maintenance treatment for non-histaminergic angioedema: analysis of efficacy and safety in 37 patients. Clin Exp Immunol 2014; 178: 112–117. DOI: 10.1111/cei.12379. PubMed Central
- Medsafe. Tranexamic acid tablets New Zealand Data Sheet. Revised 23 January 2018. Available at: http://www.medsafe.govt.nz/profs/Datasheet/t/tranexamicacidtab.pdf (accessed 1 August 2018).
On DermNet
Other websites
- Tranexamic acid tablets — Medsafe New Zealand Data Sheet
- Tranexamic acid — MyMedicines New Zealand Formulary
