Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Tralokinumab — extra information
Tralokinumab
November 2022
Author: Dr Sarah Seol, Waikato Hospital, New Zealand (2022)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction
Uses
How it works
Dosing and administration
Dosing considerations
Benefits
Side effects and risks
Contraindications
What is tralokinumab?
Tralokinumab is a fully human IgG4 monoclonal antibody used to treat moderate to severe atopic dermatitis.
In June 2021, the EU approved tralokinumab (Adtralza™) for the treatment of moderate to severe atopic dermatitis (AD) for adults in whom topical treatments are insufficient.
Tralokinumab specifically binds and neutralises the cytokine interleukin 13 (IL-13). This prevents the interaction with the IL-13 receptor and downstream inflammatory effects associated with atopic dermatitis.
It is produced using recombinant DNA technology in mouse myeloma cells.
What is tralokinumab used for?
Tralokinumab is used to treat atopic dermatitis.
Tralokinumab was also evaluated for use in several other conditions including severe asthma, idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease (COPD), and ulcerative colitis (UC), however, trials were discontinued as limited benefits were observed.
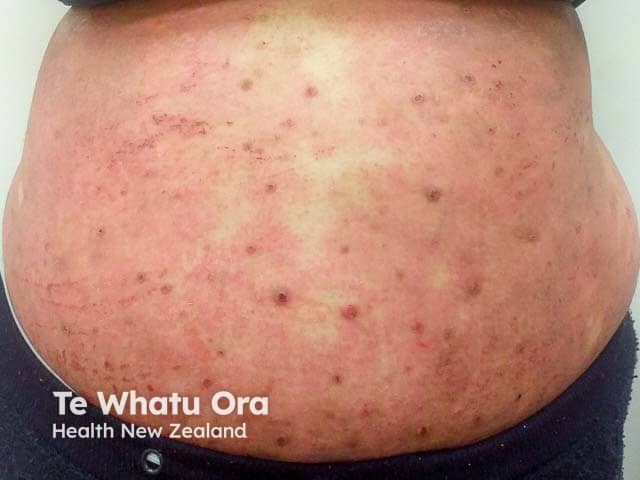
Extensive atopic dermatitis poorly controlled with emollients, potent topical steroids, and methotrexate - a potential candidate for an IL-13 antagonist
How does tralokinumab work?
Atopic dermatitis is mainly caused by an immune response involving type 2 T-helper and lymphoid cells, and downstream IL-4, IL-5, and IL-13 cytokines. Recent studies suggest IL-13 to be the most abundant cytokine in atopic dermatitis skin lesions with its levels having a direct correlation to severity of disease.
Tralokinumab binds to and neutralises IL-13, thereby stopping the downstream inflammation and disruption of skin barrier seen in AD.
Dosing and administration
- Tralokinumab is administered as a subcutaneous injection.
- For adults, an initial 600mg dose is administered, followed by 300mg every fortnight.
- For patients who achieve clear/almost clear skin, the prescribing clinician can consider dosing every four weeks.
- Consider discontinuing if no response after 16 weeks of treatment.
- Tralokinumab may be used in conjunction with topical corticosteroids.
- The pre-filled syringes should be removed from the refrigerator and left at room temperature for 30 minutes before injecting.
- Injection sites should be alternated (thigh, abdomen, or upper arms), and avoided in skin that is tender, bruised, erythematous, or damaged.
- Patients may self-inject tralokinumab once proper training has been provided.
- Missed doses should be administered as soon as possible and resumed at the regularly scheduled time thereafter.
- In the case of overdose, there is currently no specific treatment.
Dosing considerations
No dose adjustment in:
- Elderly: limited data for >75 y/olds
- Renal impairment: limited data in severe renal impairment
- Hepatic impairment: limited data in severe hepatic impairment.
Increased dosing frequency:
- Obesity: lower trough concentrations found, therefore when clear skin achieved, reducing dosage to fourth weekly may not be appropriate.
Reduced dose in:
- Paediatrics: lower doses (150mg or 300mg fortnightly) in adolescents has shown to be effective in the phase III ECTRA 6 trial. Paediatric safety and efficacy data is limited.
Limited data:
- Pregnancy: Although animal studies suggest no harmful effects, it is best to avoid tralokinumab use during pregnancy due to limited human data.
- Breastfeeding: excretion into human milk is unknown; risks and benefits of treatment should be considered.
What are the benefits of tralokinumab?
In two randomised, double-blinded, placebo-controlled, monotherapy studies (ECZTRA 1 and ECZTRA 2; 2020), and one study on the concomitant use of topical corticosteroids (ECZTRA 3; 2021), it was found that tralokinumab significantly improved patient-reported atopic dermatitis symptoms at 16 weeks, when compared to placebo.
Specifically, the number of patients achieving a 75% reduction in Eczema Area and Severity Index (EASI) score at 16 weeks, compared to control groups were as follows:
- In ECZTRA 1: 25% vs 12.7%
- In ECZTRA 2: 33.2% vs 11.4%
- In ECZTRA 3: 56.0% vs 35.7%.
ECZTRA 1 also found a reduction in Staphylococcus aureus colonisation on lesional skin following tralokinumab treatment.
What are the side effects and risks of tralokinumab?
More common (>1%) adverse effects reported during tralokinumab use include:
- Conjunctivitis
- Upper respiratory tract infections
- Injection site reactions
- Eosinophilia (transient).
Less common (<1%) reported adverse effects include:
- Keratitis
- Eczema herpeticum.
Drug interaction studies with tralokinumab, specifically whether it changes the metabolism of Cytochrome P450 (CYP) substrates in adults with moderate to severe atopic dermatitis, are currently being conducted in the ECZTRA 4 trial.
Warnings and precautions
- In the case of a systemic hypersensitivity reaction, tralokinumab administration should be discontinued immediately and treated appropriately.
- In patients who develop conjunctivitis that does not clear with standard treatment, an ophthalmology examination should be performed.
- Patients with pre-existing helminth (worm) infections were excluded from clinical trials, and it is therefore unknown how tralokinumab may affect the immune response in these patients. Helminth infections should be treated appropriately before starting tralokinumab treatment.
- The safety and efficacy of tralokinumab use with live and live attenuated vaccines have not been studied, and should therefore not be given concurrently. See also immunisations in immunosuppressed dermatology patients for more information.
What are the contraindications with tralokinumab?
Hypersensitivity to tralokinumab or any of the excipients including:
- Sodium acetate trihydrate
- Acetic acid
- Sodium chloride
- Polysorbate 80.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
Bibliography
- Duggan S. Tralokinumab: First approval. Drugs. 2021;81(14):1657–63. doi 10.1007/s40265-021-01583-1. Article
- European Medicines Agency. Adtralza. 2021. Available from: https://www.ema.europa.eu/en/documents/product-information/adtralza-epar-product-information_en.pdf
- Paller A, Blauvelt A, Soong W, Imafuku S, Hong CH, Schuttelaar ML, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN: The Journal of Cutaneous Medicine. 2022;6(2):s29. Poster
- Silverberg JI, Toth D, Bieber T, Alexis AF, Elewski BE, Pink AE, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate‐to‐severe atopic dermatitis: results from the double‐blind, randomized, multicentre, placebo‐controlled phase III ECZTRA 3 trial. British Journal of Dermatology. 2021;184(3):450–463. doi 10.1111/bjd.19573. Journal
- Wollenberg A, Blauvelt A, Guttman‐Yassky E, Worm M, Lynde C, Lacour JP, et al. Tralokinumab for moderate‐to‐severe atopic dermatitis: results from two 52‐week, randomized, double‐blind, multicentre, placebo‐controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449. doi: 10.1111/bjd.19574. Journal
- Wollenberg A, Weidinger S, Worm M, and Bieber T. Tralokinumab bei atopischer Dermatitis. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2021;19(10):1435–42. Journal
On DermNet
- Atopic dermatitis images
- Atopic dermatitis
- Barrier function in atopic dermatitis
- Treatment of atopic dermatitis
- Systemic therapy
- Biological treatments
- Immunisations in immunosuppressed dermatology patients
Other websites
- Adtralza (data sheet)
- Tralokinumab (National Eczema Foundation)
