Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Steroid impregnated tape — extra information
Steroid impregnated tape
Last reviewed: May 2023
Author: Dr Ian Coulson, Consultant Dermatologist, Lancashire, United Kingdom (2023)
Edited by the DermNet content department
What is it? Uses Contraindications Dosing and application Benefits Disadvantages Side effects and risks
What is a steroid impregnated tape?
Steroid impregnated tapes are self-adhesive plastic tapes that have a topical corticosteroid, often fludroxycortide, impregnated into the adhesive. Pieces of tape are cut accurately to cover the condition requiring treatment, allowing accurate steroid delivery to exactly where it is needed, with the added benefit of tape occlusion, which enhances the efficacy of the steroid.
Fludroxycortide tape was previously known as Haelen® tape. Steroid impregnated tapes are not available in every country worldwide.
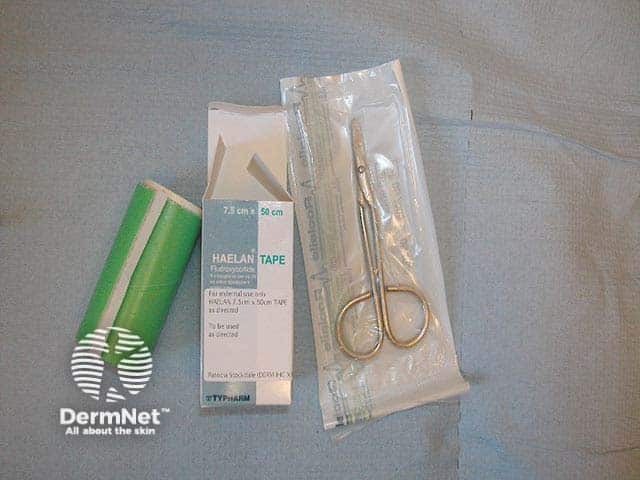
Fludroxycortide tape and clean scissors will be needed
What is steroid impregnated tape used for?
Steroid impregnated tape is used for steroid responsive localised inflammatory dermatoses where a short course of a potent topical corticosteroid is beneficial.
Examples include:
- Lichen simp
- Discoid eczema
- Prurigo
- Lichen planus
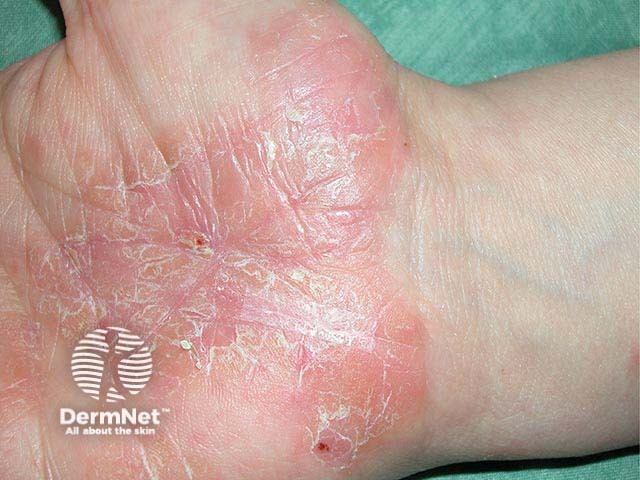
- Fissures on the hands and feet due to psoriasis or atopic dermatitis (discoid lupus erythematosus)
- Sarcoidosis of the skin
- Hypertrophic and keloid scars
- Granuloma annulare
- Necrobiosis lipoidica
- Hypergranulation.
What are the contraindications to steroid impregnated tape?
As with all topical corticosteroids, steroid impregnated tape use should be avoided on:
- Bacterial skin infections
- Viral skin infections
- Skin cancers
- Acne
- Rosacea
- Allergy to any of the constituents of the tape (both the steroid and the acrylate adhesive).
Dosing and application of steroid impregnated tape
Dosing
Most tapes contain the topical corticosteroid fludroxycortide at a concentration of 4 micrograms per square centimetre.
Preparation and application
The backing is a translucent plastic surgical tape. A protective silicone paper tape covers the adhesive and should be removed prior to application of the tape.
- Prior to application of the tape, the affected area should be washed and dried; avoid greasy emollients that may prevent the tape sticking to the skin.
- Prior to removing the paper protective tape, the dressing can be cut to size with clean scissors to accurately cover the area to be treated. A 5 mm overlap may help with adhesion and corners can be rounded off to prevent them catching on clothing.
- The sticky side of the tape can then be applied to the area to be treated. Massaging the back of the tape for a few seconds enhances the adhesion.
- It is usually left on either overnight, or for 12 hours, and then removed and discarded.
- A fresh piece of tape can be applied as above after 12 hours.
- The tape is flexible and waterproof.
Duration of treatment
The period of time required will be determined by the condition requiring treatment, guided by your dermatologist.
- Fissures on the fingers due to eczema (atopic dermatitis) or psoriasis often heal with 2 or 3 applications. Lesions of sarcoidosis and discoid lupus may need 7–14 days of treatment.
- Keloid scars can be treated for 2–4 weeks and then reassessed, and continued if the scars are flattening provided there are no adverse effects developing.
The tape has been applied to the fissure and should be left on overnight
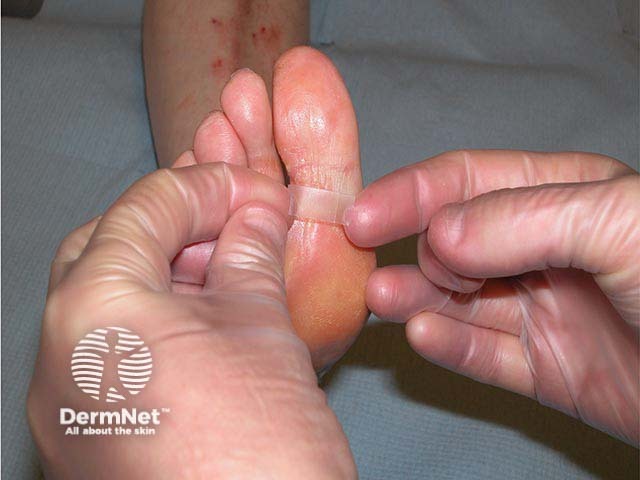
The tape can be removed after overnight application
What are the benefits of steroid impregnated tape?
The tape allows accurate localisation of the steroid and the occlusion improves its efficacy and the speed of action. It can be as effective as intralesional steroid injection, and obviously less painful to administer.
Occlusion may also minimise subconscious scratching (which can prolong and exacerbate conditions like lichen simplex and lichen planus).
What are the disadvantages of steroid impregnated tape?
The occlusive effect of the tape will allow the local adverse reactions of topical corticosteroids (see below) to occur more rapidly than an un-occluded steroid.
What are the side effects and risks of steroid impregnated tape?
In general, side effects are uncommon and localised to the area where the tape has been used. They may include:
- Burning and itching of the skin
- Steroid acne
- Folliculitis
- Depigmentation
- Hypertrichosis
- Telangiectasia
- Skin atrophy and striae
- Contact dermatitis.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
Bibliography
- Burr S. Fludroxycortide tape: A versatile, well tolerated treatment option. Dermatological Nursing. 2021;20(2):23–26. Journal
On DermNet
