Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Key clinical-trial evidence for calcipotriol/ betamethasone dipropionate foam — extra information
Key clinical-trial evidence for calcipotriol/ betamethasone dipropionate foam
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet Editor in Chief: Adjunct A/Prof Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. October 2019.
Introduction
Clinical studies
Results
Health-related quality of life
Results
Future potential
What is calcipotriol/betamethasone dipropionate foam?
Calcipotriol/betamethasone dipropionate foam (trade name Enstilar®) is a dual-action aerosol foam containing calcipotriol, a vitamin D analog, and betamethasone dipropionate, a corticosteroid. Calcipotriol is known as calcipotriene in the US.
Calcipotriol/betamethasone foam is specifically indicated for the topical treatment of plaque psoriasis in patients 18 years of age and older.
It is supplied as a foam spray for topical administration and is applied to affected areas once daily for up to 4 weeks. Treatment is discontinued when control is achieved.
The FDA approval of Enstilar foam in 2015 was based on two multicentre, randomised, double-blind trials conducted in patients with plaque psoriasis.
The product is also approved for the treatment of psoriasis in Australia, Canada, the European Union, and New Zealand, among other countries.
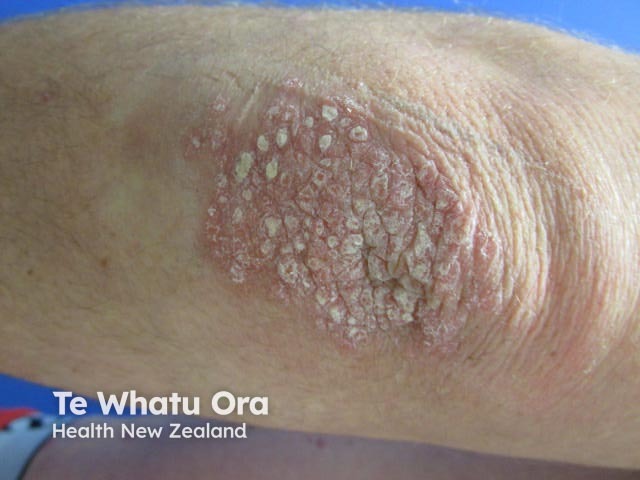
Chronic plaque psoriasis
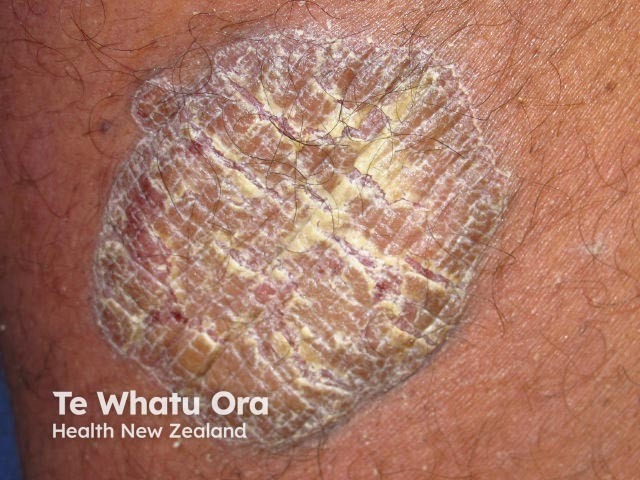
Chronic plaque psoriasis
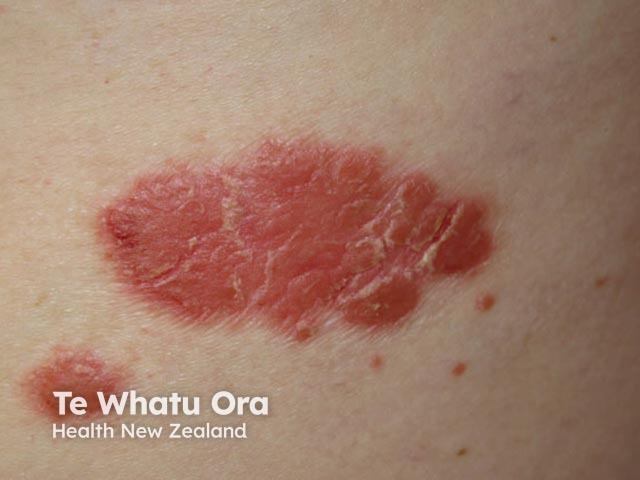
Chronic plaque psoriasis
Clinical studies
Study design
Trial 1 patients (n = 302) were randomly assigned to receive [1]:
- Calcipotriol/betamethasone foam (n = 100)
- Betamethasone dipropionate in the same vehicle (n = 101)
- Calcipotriol hydrate in the same vehicle (n = 101).
Trial 2 patients (n = 426) were randomly assigned to receive [2]:
- Calcipotriol/betamethasone foam (n = 323)
- Vehicle only (n = 103).
Disease severity was graded using a 5-point Investigator’s Global Assessment (IGA) scale. Treatment success defined as the proportion of patients at week 4 who were ‘Clear’ or ‘Almost Clear’ according to the IGA. Subjects with ‘Mild’ disease at baseline were required to be ‘Clear’ to be considered a treatment success.
The majority of subjects in both trials (76% and 75%, respectively) had a disease of moderate severity at baseline, 14% and 15% had a disease of mild severity at baseline and 10% had severe disease at baseline in both trials.
The extent of disease involvement assessed by mean body surface area was 7.1% (range 2 to 28%) and 7.5% (range 2 to 30%) in trials 1 and 2, respectively.
In both trials, patients were treated once daily for up to 4 weeks.
Results
Trial 1
The percentage of psoriasis patients achieving treatment success, according to the Investigator’s Global Assessment of Disease Severity, was 45% in the calcipotriol/betamethasone foam group, 30.7% for betamethasone dipropionate in the vehicle and 14.9% for calcipotriol in the vehicle.
Results for the primary endpoint 'treatment success' (IGA) at week 4 showed calcipotriol/betamethasone to be statistically significantly more effective than all the comparators included, and responses were observed in all categories of baseline disease severity.
The effect of calcipotriol/betamethasone on itch and itch-related sleep loss was investigated in Trial 1 using a visual analogue scale (VAS) ranging from 0 mm (no itch/no sleep loss at all) to 100 mm (worst itch you can imagine/worst possible sleep loss).
A statistically significantly higher number of patients in the calcipotriol/betamethasone group compared to vehicle achieved a 70% reduction in itch and itch-related sleep loss from day 3 and throughout the treatment period.
Trial 2
The percentage of psoriasis patients achieving treatment success according to the Investigator’s Global Assessment of Disease Severity was 53.3% in the calcipotriol/betamethasone foam group and 4.8% in the vehicle-only group.
In trial 2, the effect of calcipotriol/betamethasone on scalp psoriasis was investigated as the percentage of patients with 'treatment success' according to the PGA of the scalp at Week 4.
The percentage of subjects with 'treatment success' according to the PGA of the scalp at Week 4 was 53% in the calcipotriol/betamethasone foam group, 47.5% in the betamethasone dipropionate in the vehicle group, and 35.6% in the calcipotriol in the vehicle only group.
Calcipotriol/betamethasone foam was statistically significantly more effective compared to calcipotriol and also associated with a higher rate of treatment success than betamethasone dipropionate, but this comparison did not reach statistical significance.
Adverse reactions — trials 1 and 2
The estimation of the frequency of adverse reactions is based on a pooled analysis of data from clinical studies.
Adverse effects associated with the use of calcipotriol/betamethasone foam include the following:
- Application site irritation (uncommon ≥1/1,000 to <1/100)
- Application site pruritus (uncommon ≥1/1,000 to <1/100)
- Folliculitis (uncommon ≥1/1,000 to <1/100)
- Hypopigmentation (uncommon ≥1/1,000 to <1/100)
- Hypercalcemia (uncommon ≥1/1,000 to <1/100)
- Urticaria
- Exacerbation of psoriasis (uncommon ≥1/1,000 to <1/100).
Health-related quality of life
Health-related quality of life (HRQoL) measures provide patient-centred evaluations of response to treatment.
In the 12-week, Phase III PSO-ABLE study, fixed-combination calcipotriol 50 μg/g as hydrate (Cal) plus betamethasone 0.5 mg/g as dipropionate (BD) aerosol foam was significantly more effective for the treatment of psoriasis than Cal/BD gel [3].
HRQoL was assessed using the Dermatology Life Quality Index (DLQI), EuroQoL, and Psoriasis QoL (PQoL-12) questionnaires at baseline and weeks 4, 8, and 12.
Itch, itch-related sleep loss, and work impairment were also assessed.
Study design
463 patients were randomised to Cal/BD foam (n = 185), Cal/BD gel, (n = 188) foam vehicle (n = 47), and gel vehicle (n = 43).
Results
Significantly more Cal/BD foam patients achieved DLQI scores of 0/1 at weeks 4 (45.7% vs 32.4%; p = 0.013) and 12 (60.5% vs 44.1%; p = 0.003) than Cal/BD gel patients.
Cal/BD foam significantly improved EQ-5D utility index (0.09 vs 0.03; p < 0.001) and PQoL-12 scores (-2.23 vs -2.07; p = 0.029) from baseline to week 4 versus Cal/BD gel.
Itch, itch-related sleep loss and work impairment improved more with Cal/BD foam than gel.
What is the future potential for calcipotriol/betamethasone foam?
- Fixed-combination calcipotriol 50 μg/g plus betamethasone dipropionate 0.5 mg/g (Cal/BD) aerosol foam is a new topical treatment for psoriasis.
- It is rapidly effective, offers greater efficacy compared to an equivalent gel formulation, and has been shown to increase patient treatment satisfaction.
- Topical foam vehicles are innovative alternatives to creams and ointments, addressing some of the patient challenges experienced with traditional vehicles.
- Well-designed foam vehicles are easily spread over large areas of the skin and do not leave a greasy or oily film on the skin after application.
- Although moderate-to-severe psoriasis is typically treated with systemic or biological therapies, an effective topical treatment may be a cost-saving alternative or adjunct to systemic therapy.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Lebwohl M, Tyring S, Bukhalo M, et al. Fixed combination aerosol foam calcipotriene 0.005% (Cal) plus betamethasone dipropionate 0.064% (BD) is more efficacious than Cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double-blind, multicenter, three-arm, phase 2 study. J Clin Aesthet Dermatol. 2016; 9: 34–41. PubMed
- Leonardi C, Bagel J, Yamauchi P, et al. Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris ‐ a randomized phase III study (PSO‐FAST). J Drugs Dermatol. 2015; 14: 1468–77. PubMed
- Griffiths CE, Stein Gold L, Cambazard F, et al. Greater improvement in quality of life outcomes in patients using fixed-combination calcipotriol plus betamethasone dipropionate aerosol foam versus gel: results from the PSO-ABLE study. Eur J Dermatol. 2018; 28: 356–63. PubMed
On DermNet
- Guidelines for the management of psoriasis
- Chronic plaque psoriasis
- Calcipotriol
- Topical corticosteroid
Other websites
- Enstilar foam — US FDA prescribing information PDF [accessed August 2019]
