Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Key clinical-trial evidence about dupilumab — extra information
Key clinical-trial evidence about dupilumab
Author: Anoma Ranaweera B.V.Sc; PhD (Clinical Biochemistry, University of Liverpool, UK). Chief Editor: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, January 2017.
Introduction
Evidence of efficacy from trials
Adverse reactions
Next steps
Introduction
There have been three pivotal phase 3 clinical studies evaluating dupilumab in the treatment of atopic dermatitis.
Dupilumab (Dupixent™; Sanofi, Paris, France; Regeneron, New York, USA) has shown significant efficacy and a favourable safety profile in two pivotal Phase 3 studies in monotherapy for moderate-to-severe atopic dermatitis, and in concomitant administration with topical corticosteroids.
The US Food and Drug Administration (FDA) has granted dupilumab a breakthrough therapy designation; The Biologics License Application (BLA) for dupilumab was recently accepted for Priority Review by the FDA with a target action date of March 29, 2017.
Dupilumab inhibits signalling of interleukin (IL)-4 and IL-13, two key cytokines required for the type 2 (including Th2) immune response, which is believed to be a major driver in atopic dermatitis.
Evidence for dupilumab efficacy from SOLO 1 and SOLO 2 trials
- FDA approval of dupilumab will be based on efficacy and safety results from three phase 3 studies (SOLO 1 & 2 and CHRONOS) in the global LIBERTY Atopic Dermatitis programme that included more than 2,500 patients.
- The goal of the studies was to evaluate dupilumab as monotherapy (SOLO 1 and SOLO 2) and in concomitant administration with topical corticosteroids (CHRONOS), in adult patients with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies.
- SOLO 1 and SOLO 2 trials were identical, multinational, and double-blind and included 671 and 708 adults, respectively, with atopic dermatitis for whom topical medications were contraindicated or did not provide adequate control.
- Patients were randomly assigned in a 1:1:1 ratio to receive, for 16 weeks, subcutaneous dupilumab (300 mg) or placebo weekly or the same dose of dupilumab every other week alternating with placebo.
- Patients presented with moderate-to-severe disease, with a median 50% Body Surface Area (BSA) affected by atopic dermatitis and median disease duration of 25 years.
- The use of topical agents for atopic dermatitis was not permitted except as rescue therapy for uncontrolled symptoms.
- At baseline, the patients had an Investigator Global Assessment (IGA) score of 3 or greater and an Eczema Area and Severity Index (EASI) score of 16 or higher.
- The primary endpoint was a score of clear or almost clear – 0 or 1 – on the Investigator’s Global Assessment (IGA) at week 16 accompanied by a reduction of at least 2 points from baseline. A key secondary endpoint was at least a 75% improvement in the Eczema Area and Severity Index (EASI-75), considered a coprimary endpoint by regulators in Japan and the European Union.
- In SOLO 1, the primary outcome was achieved in 85 patients (38%) who received dupilumab every other week and in 83 (37%) who received dupilumab weekly, as compared with 23 (10%) who received placebo (p < 0.001 for both comparisons with placebo).
- The results were similar in SOLO 2, with the primary outcome occurring in 84 patients (36%) who received dupilumab every other week and in 87 (36%) who received dupilumab weekly, as compared with 20 (8%) who received placebo (p < 0.001 for both comparisons).
- In addition, in the two trials, an improvement from baseline to week 16 of at least 75% on the Eczema Area and Severity Index was reported in significantly more patients who received each regimen of dupilumab than in patients who received placebo (p < 0.001 for all comparisons).
- Table 1 provides a summary of the efficacy of dupilumab in SOLO 1 and 2 clinical trials.
Table 1. Improvements in atopic dermatitis at 16 Weeks
Placebo |
Dupilumab every 2 weeks |
Dupilumab weekly |
P value vs placebo |
|
IGA (SOLO 1) |
10% |
38% |
37% |
0.0001 |
IGA (SOLO 2) |
9% |
36% |
36% |
0.0001 |
EASI-75 (SOLO 1) |
15% |
51% |
53% |
0.0001 |
EASI-75 (SOLO 2) |
12% |
44% |
48% |
0.0001 |
- Dupilumab was also associated with improvement in secondary clinical end points, including reduction in pruritus and symptoms of anxiety or depression and improvement in quality of life.
- The reduction in the daily intensity of patient-reported itch, as measured by the Pruritus Numerical Rating Scale (NRS), was a secondary endpoint that was met at 2 weeks, 4 weeks and 16 weeks.
- The Pruritus NRS ranges from 0 (no itch) to 10 (worst itch imaginable).
- At 16 weeks, for SOLO 1 and SOLO 2, respectively, 41 and 36 percent of patients who received dupilumab 300 mg every two weeks, and 40 and 39 percent of patients who received dupilumab 300 mg weekly, achieved a four-point or greater reduction in their NRS score compared to 12 and 10 percent with placebo (p < 0.001 for all regimens and trials).
- Participants completed the Hospital Anxiety and Depression Scale (HADS) at baseline and 16 weeks.
- On the HADS total, placebo patients improved 3.0 points compared with 5.2 points for both dupilumab groups in SOLO 1 (p = 0.0006 twice weekly dupilumab, p = 0.0003 weekly dupilumab).
- In SOLO 2, placebo patients improved 0.8 points compared with 5.1 and 5.8 points in the dupilumab twice weekly and weekly groups, respectively (both p < 0.0001).
- Injection-site reactions and conjunctivitis were more frequent in the dupilumab groups than in the placebo groups.
- The median baseline Dermatology Life Quality Index score was 15 across the two parallel trials. The collective proportion of patients who experienced at least a 4-point improvement, which is considered a clinically meaningful response, was 29.1% in controls, compared with 68.6% in patients dupilumab every other week and 60.2% with weekly dupilumab.
- In conclusion, in two phase 3 trials of identical design involving patients with atopic dermatitis, dupilumab improved the signs and symptoms of atopic dermatitis, including pruritus, symptoms of anxiety and depression, and quality of life, as compared with placebo. Trials of longer duration are needed to assess the long-term effectiveness and safety of dupilumab.
Adverse reactions: clinical trial experience
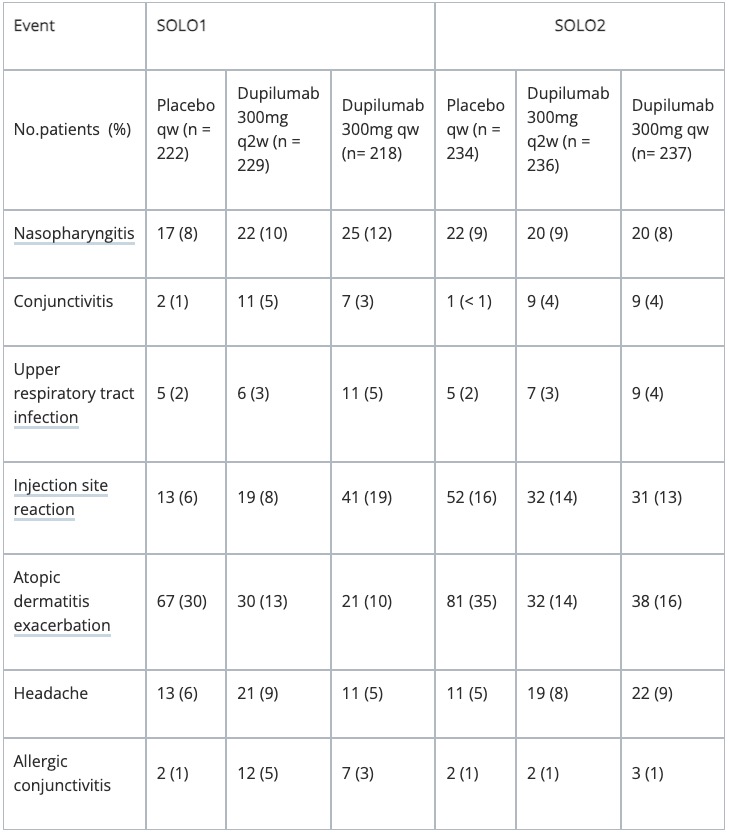
- Adverse events observed in > 5% of patients participating in key clinical trials of dupilumab are summarised in the table below.
Long-term follow-up – clinical trial evidence from CHRONOS
- Dupilumab used with topical corticosteroids (TCS) was superior to treatment with TCS alone in long-term (1 year) phase 3 trial (LIBERTY AD CHRONOS) in inadequately controlled moderate-to-severe atopic dermatitis patients.
- A total of 740 adult patients with moderate-to-severe atopic dermatitis were enrolled in CHRONOS.
- All patients were inadequately controlled with topical medications and were assessed via the 5-point Investigator's Global Assessment (IGA) scale, ranging from 0 (clear) to 4 (severe); entry criteria required a baseline score of 3 or 4.
- Patients were also assessed using the Eczema Area and Severity Index (EASI) and other measures.
- All patients initiated daily treatment with a medium potency TCS or low potency TCS on areas of the body where medium potency TCS is considered unsafe.
- Patients were randomised in a 3:1:3 fashion into the following treatment groups (all in combination with TCS): dupilumab 300 mg subcutaneously once per week (n=319), dupilumab 300 mg subcutaneously every two weeks (n=106), or placebo (n=315).
- This design allowed sufficient power for the efficacy endpoints in both dupilumab groups while increasing the available safety data on the more frequent dosing regimen.
Co- primary endpoint results at week 16 were:
- 39 percent of patients who received either dupilumab 300 mg weekly with TCS or dupilumab 300 mg every two weeks with TCS achieved clearing or near-clearing of skin lesions (IGA 0 or 1), compared to 12 percent of patients receiving placebo with TCS (p < 0.0001).
- 64 percent of patients who received dupilumab 300 mg weekly with TCS, and 69 percent of patients who received dupilumab 300 mg every two weeks with TCS achieved EASI-75, a 75 percent reduction on an index measuring eczema severity, compared to 23 percent of patients receiving placebo with TCS (p < 0.0001).
Co-primary end-point results at week 52 were:
- 40 percent of patients who received dupilumab 300 mg weekly with TCS, and 36 percent of patients who received dupilumab 300 mg every two weeks with TCS achieved clearing or near-clearing of skin lesions (IGA 0 or 1), compared to 12.5 percent of patients receiving placebo with TCS (p <0.0001).
- 64 percent of patients who received 300 mg weekly with TCS, and 65 percent of patients who received 300 mg every two weeks with TCS achieved EASI-75, compared to 22 percent with placebo with TCS (p < 0.0001).
- The overall rate of adverse events was comparable between the dupilumab with TCS groups (83 percent for the weekly dose (qw) and 88 percent for the every two weeks (q2w) dosing group) and the placebo with TCS group (84 percent).
- Adverse events that were noted to have a higher rate with dupilumab included injection site reactions (20 (qw) and 16 percent (q2w) dupilumab; 9 percent placebo) and conjunctivitis (19 (qw) and 13 (q2w) percent dupilumab; 8 percent placebo); 22 percent of patients on placebo, and 23 (qw) and 28 percent (q2w) of patients on dupilumab reported a history of allergic conjunctivitis at study entry.
Next steps with dupilumab
- Dupilumab is an innovative first-in-class investigational agent that has shown significant efficacy and a favourable safety profile in two pivotal Phase 3 studies in monotherapy for moderate-to-severe atopic dermatitis (SOLO 1 & SOLO 2), and in concomitant administration with topical corticosteroids (CHRONOS).
- There were clinically meaningful and significant improvements in skin clearing (IGA scores) disease severity (EASI scores), symptoms (pruritus NRS) and health-related quality of life.
- The toxicities identified were manageable and reversible upon discontinuation of treatment.
- Trials of longer duration and trials including patients with severe disease only and ineligible for systemic immunosuppressants are needed to further assess effectiveness and safety of dupilumab.
- According to the manufacturer, Regeneron, pivotal phase 3 studies of dupilumab in paediatric atopic dermatitis in children aged 6 months to 17 years will begin in 2017.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016 Dec 15;375(24):2335–48. PubMed.
- Simpson EL, Gadkari A, Worm M, Soong W, et al. Dupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): A phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD). J Am Acad Dermatol. 2016 Sep; 75(3):506–15. PubMed.
- Simpson EL, Bieber T, Eckert L, Wu R, et al. Patient burden of moderate to severe atopic dermatitis (AD): Insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016 Mar;74(3):491–8. PubMed.
- Thaçi D, Simpson EL, Beck LA, Bieber T, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016 Jan 2;387(10013):40–52. PubMed.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. Lancet. 2016 Jan 2; 387(10013):4–5. PubMed.
- Hamilton JD, Suárez-Fariñas M, Dhingra N, Cardinale I, Li X, Kostic A et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014 Dec; 134(6):1293–300. PubMed.
On DermNet
