Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Cemiplimab — extra information
Treatments Lesions (cancerous)
Cemiplimab
Author: Anoma Ranaweera, Medical Writer, Auckland, New Zealand. DermNet Editor in Chief: Adjunct A/Prof Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell/Maria McGivern. November 2018.
Introduction How it works How to use Potential drug interactions Adverse events Risks Uses in specific populations
What is cemiplimab?
Cemiplimab (also called cemiplimab-rwlc; trade name Libtayo®) is a prescription medicine used to treat people with cutaneous squamous cell carcinoma (SCC).
In April 2018, the European Medicines Agency (EMA) accepted for review the marketing authorisation application for cemiplimab for the treatment of patients with metastatic cutaneous SCC or locally advanced cutaneous SCC who are not candidates for surgery.
In September 2018, the US Food and Drug Administration (FDA) approved cemiplimab as a breakthrough therapy for the treatment of patients with metastatic cutaneous SCC or locally advanced SCC who are not candidates for curative surgery or curative radiation. Regeneron and Sanofi-Aventis (New Jersey, USA) will market Libtayo® jointly in the US.
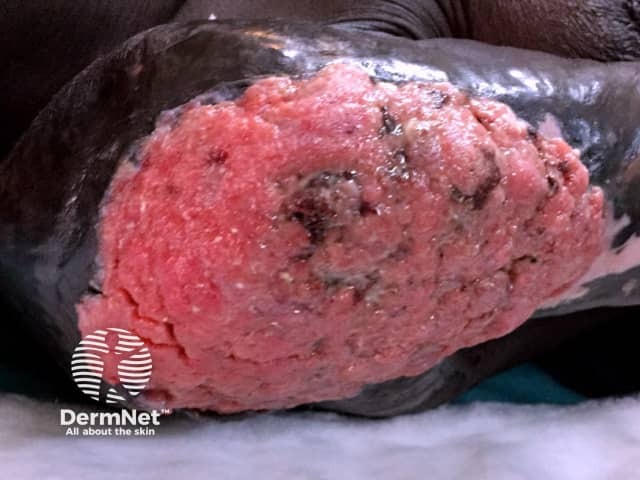
Advanced squamous cell carcinoma arising in thermal burn scar
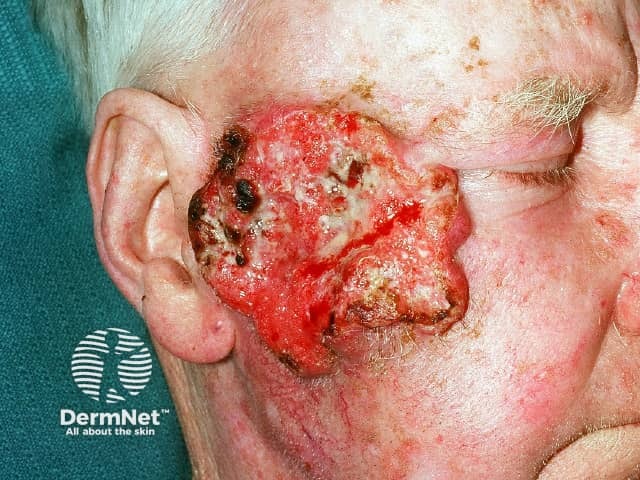
Advanced squamous cell carcinoma
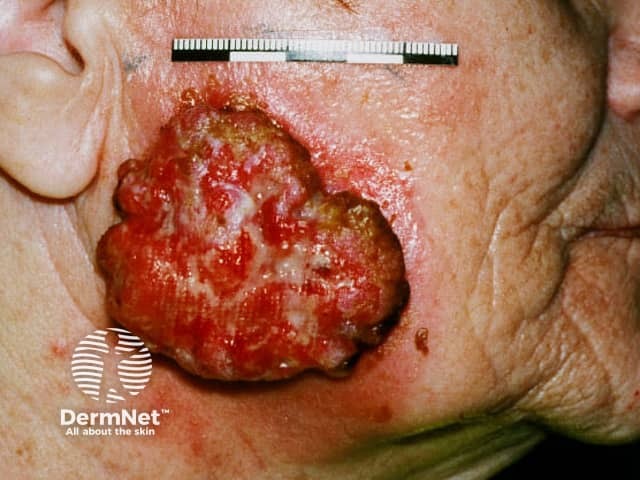
Advanced squamous cell carcinoma
Cemiplimab is the first and only treatment specifically approved and available for advanced cutaneous SCC in the US. This drug was evaluated by the FDA under priority review, which is reserved for medicines that represent significant improvements in safety or efficacy in treating serious conditions.
The EMA review process is anticipated to be complete in the first half of 2019. There are currently no EMA-approved treatments for advanced cutaneous SCC.
Cemiplimab is under investigation in other malignancies. It is in Phase III development for cervical cancer and basal cell carcinoma, and in Phase II development for other cancers including glioblastoma multiforme, ovarian and prostate cancers, and acute lymphocytic leukaemia.
Regulatory applications in additional countries are currently being considered for submission. Cemiplimab is currently not available in New Zealand.
How does cemiplimab work?
Cemiplimab is a highly selective humanised monoclonal immunoglobulin G4 (IgG4) antibody that targets checkpoint inhibitor programmed cell death protein 1 (PD-1) and ligand 2 (PD-L2), releasing the PD-1 pathway-mediated inhibition of the immune response. This includes the anti-tumour immune response, thereby decreasing tumour growth.
- The PD-1 death receptor is a surface molecule expressed on antigen-stimulated T cells.
- When unbound, the PD-1 receptor acts as an immune checkpoint receptor enabling self-tolerance by T cells; thus, preventing autoimmune reactions.
- However, the binding of PD-1 to its ligands, programmed cell death ligand 1 (PD-L1) and ligand 2 (PD-L2), suppresses this immune response by inducing downstream signalling that inhibits the proliferation of T cells, cytokine release, and cytotoxicity.
- Abnormal PD-L1 expression on the surface of squamous carcinoma cells activates PD-1 and suppresses cytotoxic T-cell activity.
- This T-cell tolerance enables the tumour cells to avoid recognition and attack by the immune system.
How is cemiplimab administered?
The recommended dosage of cemiplimab is 350 mg as an intravenous infusion over 30 minutes every 3 weeks until disease progression decreases or unacceptable toxicity is reached.
Dosage modification
Severe and fatal immune-mediated adverse reactions may necessitate the withholding or discontinuing of cemiplimab.
Cemiplimab should be withheld in the following conditions:
- Grade 2 pneumonitis
- Grades 2 or 3 colitis
- Hepatitis (aspartate transaminase [AST] or alanine [ALT]) increased >3 and ≤10 times the upper limit of normal [ULN], or total bilirubin increased ≤3 times the upper limit of normal)
- Grades 2–4 endocrinopathies (withhold if clinically necessary)
- Other immune-mediated Grade 3 adverse reactions involving a major organ.
Cemiplimab should be permanently discontinued in the following conditions:
- Grades 3 or 4 pneumonitis
- Grade 4 colitis
- Hepatitis (AST or ALT increased >10 times the ULN or total bilirubin increased >3 times the ULN)
- Grades 3 or 4 infusion-related reactions
- Recurrent or persistent immune-mediated adverse reactions (persistent for ≥ 12 weeks after the last dose or requiring prednisone ≥ 10 mg/day or the equivalent for ≥12 weeks after last cemiplimab dose).
Renal impairment
No dosage adjustment is necessary for mild or moderate renal impairment as no clinically important effect on the exposure of cemiplimab has been observed in pharmacokinetic population studies.
Hepatic impairment
No dosage adjustment is necessary for mild hepatic impairment as no clinically important effect on the exposure of cemiplimab has been observed. There are no studies in patients with moderate or severe hepatic impairment.
What are potential drug interactions with cemiplimab?
No formal pharmacokinetic drug interaction studies have been conducted with cemiplimab.
What are the possible adverse events of cemiplimab?
In clinical trials, the most common adverse events (≥ 10%) in patients receiving cemiplimab up to 3 mg/kg every 2 weeks were:
- Fatigue
- Nausea
- Pruritus (itch)
- Rash (unspecified)
- Decreased appetite
- Constipation
- Diarrhoea.
The most frequent (≥2%) serious adverse drug reactions observed with cemiplimab were urinary tract infection, sepsis, pneumonia, pneumonitis, and cellulitis. Cemiplimab can also cause infusion reactions, including anaphylaxis.
Severe and fatal immune-mediated adverse drug reactions
- Cemiplimab binds to the PD-1 receptor, blocking the PD-1/PD-L1 pathway, thereby removing inhibition of the immune response, with the potential for breaking peripheral tolerance (the managing of T and B cells to prevent autoimmune reactions) and inducing of immune-mediated adverse reactions.
- Immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, hypophysitis (inflammation of the pituitary gland), hypothyroidism or hyperthyroidism, type 1 diabetes mellitus, and nephritis have been reported during clinical trials.
- Other immune-mediated adverse reactions involving other systems (eg, neurological, cardiovascular, and ocular reactions) were also reported in under 1% of patients in clinical trials.
- Clinical chemistry tests, including liver tests and thyroid function tests, should be done at baseline and periodically during treatment and medical management instituted promptly if required.
Skin problems due to cemiplimab
Signs of these skin problems may include:
- Rash
- Itching
- Blistering
- Painful ulcers in the mouth, nose, throat, and genital area.
What are the risks with cemiplimab?
Before receiving treatment with cemiplimab, the healthcare provider should be informed if the patient has any of the following characteristics:
- Immune system problems (eg, Crohn disease, ulcerative colitis, or systemic lupus erythematosus)
- A history of organ transplantation
- Lung or breathing problems
- Liver or kidney problems
- A history of diabetes
- Is pregnant or plans to become pregnant
- Is breastfeeding or plans to breastfeed.
The use of cemiplimab in specific populations
Pregnant women
- Based on its mechanism of action, cemiplimab can cause fetal harm when administered to a pregnant woman.
- Human IgG4 immunoglobulins are known to cross the placenta; therefore, cemiplimab has the potential to be transmitted from the mother to the developing fetus.
- Animal studies have demonstrated that inhibition of the PD-1/PD-L1 pathway can lead to increased risk of immune-mediated rejection of the developing fetus resulting in fetal death.
- There are no available data on the use of cemiplimab in pregnant women.
Nursing mothers
It is not known whether cemiplimab or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, mothers should discontinue nursing prior to using cemiplimab.
Females of reproductive potential
Females of reproductive potential should be advised to use effective contraception during treatment with cemiplimab and for at least 4 months after the last dose of the drug.
Children
The safety and effectiveness of cemiplimab have not been established in children.
Older people
In clinical studies, of the 163 patients with metastatic and locally advanced cutaneous SCC who received cemiplimab, 72% were 65 years or older and 37% were 75 years or older. No overall differences in safety or effectiveness were observed between these and younger patients.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Papadopoulos KP, Owonikoko TK, Johnson ML, et al. REGN2810: A fully human anti-PD-1 monoclonal antibody, for patients with unresectable locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC) — Initial safety and efficacy from expansion cohorts (ECs) of phase I study. J Clin Oncol 2017; 35 (15 Suppl): Abstract 9503. DOI: 10.1200/JCO.2017.35.15_suppl.9503. Journal
- Rischin CD, Migden MR, Chang A, et al. Primary analysis of phase 2 results for cemiplimab, a human monoclonal anti-PD-1, in patients with metastatic cutaneous squamous cell carcinoma (mCSCC). J Clin Oncol 2018; 36 (15 Suppl): Abstract 9519. DOI: 10.1200/JCO.2018.36.15_suppl.9519. Journal
- Migden MR, Rischin CD, Schmults A, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018; 379: 341–51. DOI: 10.1056/NEJMoa1805131. PubMed
On DermNet
- Key clinical-trial evidence for cemiplimab
- Cutaneous squamous cell carcinoma
- Cutaneous squamous cell carcinoma in skin of colour
- Biological treatments
Other websites
- LIBTAYO® (cemiplimab – rwlc) for injection for intravenous use — US Food and Drug Administration [prescribing information, September 2018]
- Cemiplimab — Medscape [data sheet]
- Diagnosis and treatment of cutaneous squamous cell carcinoma — European Journal of Cancer [European guidelines for treating cutaneous squamous cell carcinoma]
- Squamous cell carcinoma — British Association of Dermatologists [patient information]
- Squamous cell carcinoma — American Academy of Dermatology
- Optimal care pathway for people with basal cell carcinoma or squamous cell carcinoma — Cancer Council of Australia
