Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Berdazimer — extra information
Berdazimer
March 2023
Author(s): Dr Tara Sholji, NHS Lothian, Scotland (2023)
Reviewing dermatologist: Dr Ian Coulson
Edited by the DermNet content department
Introduction Uses Mechanism Application Contraindications Pharmacokinetics Benefits Disadvantages Side effects and risks
What is berdazimer?
Berdazimer is a first-in-class nitric oxide (NO)-releasing topical treatment under evaluation for use in acne and molluscum contagiosum.
What is berdazimer used for?
Berdazimer 10.3% gel is currently being reviewed by the FDA for the treatment of molluscum contagiosum. Potential FDA approval is anticipated for early 2024.
Berdazimer 3.4% gel is currently in Phase III trials for the treatment of acne.
Typical pearl-like mollusca suitable for berdazimer
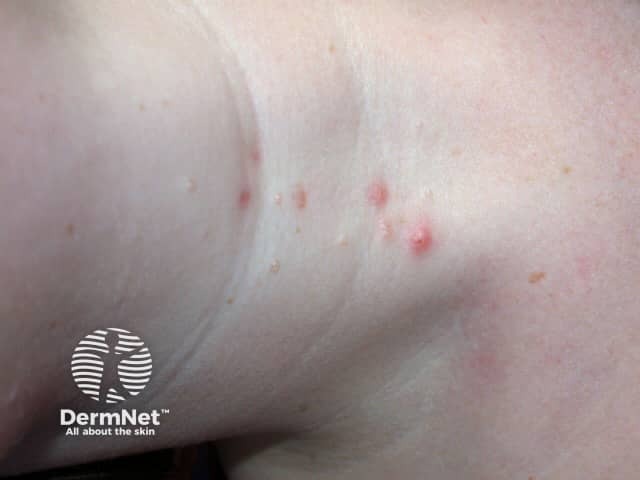
Molluscum contagiosum
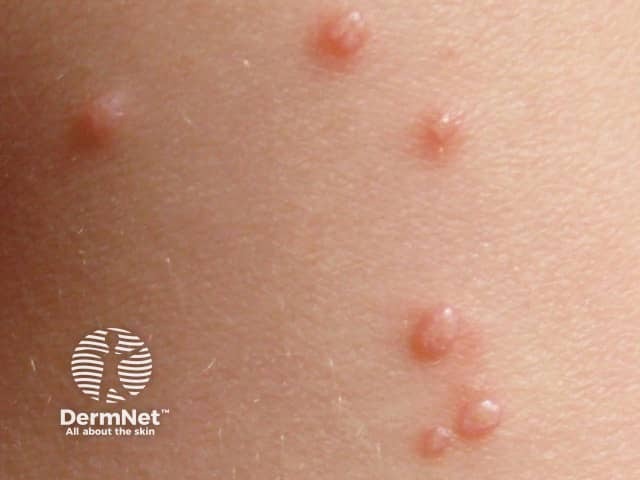
Molluscum contagiosum
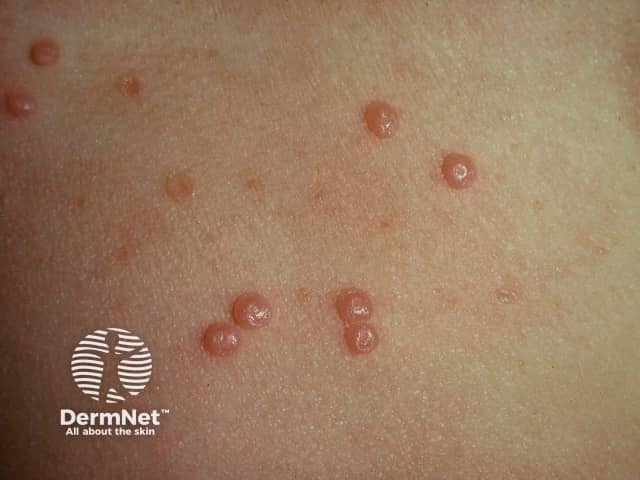
Molluscum contagiosum
How does berdazimer work?
Nitric oxide (NO) is naturally produced by the human body and has multiple functions including immune defence. It acts as an antimicrobial via protein nitrosylation that affects immunomodulation, apoptosis, cytokine production, and inflammation. NO can also affect viral replication through cytotoxic reactive oxygen and nitrogen molecules.
Berdazimer consists of two components that act to promote nitric oxide (NO) release:
- The nitric oxide donor, berdazimer sodium
- A hydrogel which acts as a proton donor on the skin and encourages the controlled release of NO.
How to use berdazimer
During trials for molluscum contagiosum, a thin layer of berdazimer 10.3% gel was applied once daily to the lesions.
What are the contraindications with berdazimer?
Hypersensitivity to berdazimer or any of its constituents; otherwise no specific known contraindications have yet been identified.
Pharmacokinetics
Measurement of NO metabolite hMAP3 during the Phase I study showed that once-daily application of berdazimer resulted in minimal systemic absorption in children aged 2–12 years old.
What are the benefits of berdazimer?
The short half-life of unstable gas nitric oxide (NO) and its immunomodulating effects have previously been difficult to use therapeutically, due to challenges with storage and targeted release to treatment sites.
Berdazimer, a first-in-class gel, allows self-application along with the stable release of NO to the affected skin at the time and site of application, minimising systemic exposure.
This is significant as a potential alternative to current molluscum contagiosum treatments including in-clinic curettage, cryotherapy, laser ablation, or chemical removal of the lesions.
In B-SIMPLE4 (a Phase III multi-centre, double-blind, vehicle-controlled, randomised controlled trial), 891 patients aged ≥6 months with 3–70 molluscum contagiosum lesions were randomly assigned to the berdazimer gel or vehicle group for 12 weeks.
- Participant-reported complete clearance in the berdazimer group was 37% (145/391) at week 12 and 60% (226/374) at week 24, compared with 20% (79/390) of the vehicle group at week 12 and 46% (174/378) at week 24. These values were similar to investigator-reported clearance rates.
- There was also a 27% reduction in the mean number of lesions by week 4 of berdazimer treatment, compared to 5.6% reduction in the vehicle group.
What are the disadvantages of berdazimer?
- Unknown efficacy in sexually transmitted molluscum contagiosum, lesions in the periocular area, and those with extensive dermatitis surrounding lesions (excluded from the B-SIMPLE4 trial).
- Unknown effect of concomitant use with other topical treatments.
- Can take up to 12 weeks for full clearance of lesions, and sometimes longer.
- Efficacy and safety data for use in molluscum contagiosum is so far limited to a maximum of 12 weeks of use.
What are the side effects and risks of berdazimer?
In the B-SIMPLE4 trial, berdazimer was generally well tolerated.
Application site reactions (eg, pain, erythema, itching, burning, or stinging) were the most common side effects and mostly mild to moderate; they were the main reason for treatment discontinuation. Overall, these reactions peaked around week 2 of treatment and decreased over the remainder of the study.
No systemic adverse events were observed.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
Bibliography
- Browning JC, Cartwright M, Thorla I, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 clinical trial patients and caregivers: Global impression of change and exit interview substudy results. Am J Clin Dermatol. 2023;24:119–133. doi: 10.1007/s40257-022-00733-9. Journal
- Browning JC, Enloe C, Cartwright M, et al. Efficacy and safety of topical nitric oxide−releasing berdazimer gel in patients with molluscum contagiosum. JAMA Dermatology. 2022;158(8):871–878. doi: 10.1001/jamadermatol.2022.2721. Journal
- Cartwright M, Enloe C, Stripling S, Maeda-Chubachi T. Pharmacokinetic profile, safety, and tolerability of topical Berdazimer gel, 10.3% in patients with molluscum contagiosum. J Drugs Dermatol. 2022;21(10):1104–1110. doi: 10.36849/jdd.6938. Journal
- Hebert AA, Siegfried EC, Durham T, et al. Efficacy and tolerability of an investigational nitric oxide–releasing topical gel in patients with molluscum contagiosum: A randomized clinical trial. J Am Acad Dermatol. 2020;82(4):887–894. doi: 10.1016/j.jaad.2019.09.064. Journal
- Stafford PB. Form 10-K Novan, Inc. United States Securities and Exchange Commission. Published February 24, 2020. Accessed January 22, 2023. Document
On DermNet
Other websites
- Molluscum contagiosum — Centres for Disease Control and Prevention (CDC)
- Berdazimer gel — Novan
