Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Symmetrical drug-related intertriginous and flexural exanthema — extra information
Symmetrical drug-related intertriginous and flexural exanthema
Authors: Dr Mark Duffill, Dermatologist, Hamilton, New Zealand, 2008; revised Hon A/Prof Amanda Oakley Chief Editor, 2015; Updated by: Dr Ayesha Vos, Junior Medical Officer, Eastern Health, Melbourne, Victoria, Australia; A/Prof Rosemary Nixon AM, Dermatologist, The Skin Institute, Carlton, Victoria, Australia. Copy edited by Gus Mitchell. January 2021.
Introduction
Demographics
Causes
Clinical features
Complications
Diagnosis
Differential diagnoses
Treatment
Outcome
Appendix: What drugs are reported to cause symmetrical drug-related intertriginous and flexural exanthema?
What is symmetrical drug-related intertriginous and flexural exanthema?
Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an adverse drug eruption presenting as a distinctive erythematous rash involving the skin folds. It was initially called ‘baboon syndrome’ as the buttocks were predominantly affected in the first patients described with this reaction, although this may be an unrelated entity caused by sensitisation to a topical agent followed by systemic exposure.
Who gets symmetrical drug-related intertriginous and flexural exanthema?
Symmetrical drug-related intertriginous and flexural exanthema can affect all age groups, but is rare in children. SDRIFE shows a male predominance of 3:1.
What causes symmetrical drug-related intertriginous and flexural exanthema?
Symmetrical drug-related intertriginous and flexural exanthema is a type IV delayed hypersensitivity reaction to a systemic drug [see Allergies explained]. The reason for the characteristic distribution of the rash is not known.
Amoxicillin and other beta-lactam antibiotics (penicillins, cephalosporins) are the most common cause of SDRIFE, accounting for approximately 50% of cases. A detailed list of medications reported in association with SDRIFE is found in the appendix.
What are the clinical features of symmetrical drug-related intertriginous and flexural exanthema?
Symmetrical drug-related intertriginous and flexural exanthema appears hours to days after exposure to the medication. The rash presents as a well-defined redness of the buttocks, natal cleft, and/or upper inner thighs, resembling the red bottom of baboons. The redness is usually symmetrical and often forms a V-shape. The neck, axillae, and other large skin folds (flexures) may also be involved.
Rare clinical features include pustules, vesicles/bullae, petechiae/purpura, and oral mucosal lesions.
A key feature is the lack of systemic symptoms. The affected person is not unwell and the rash is not accompanied by any other symptoms.

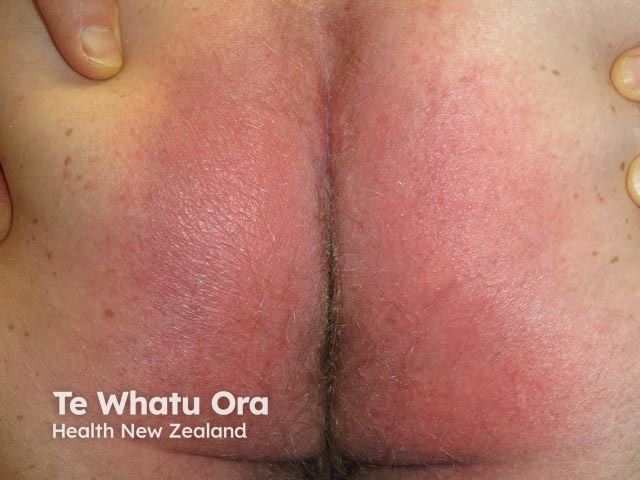
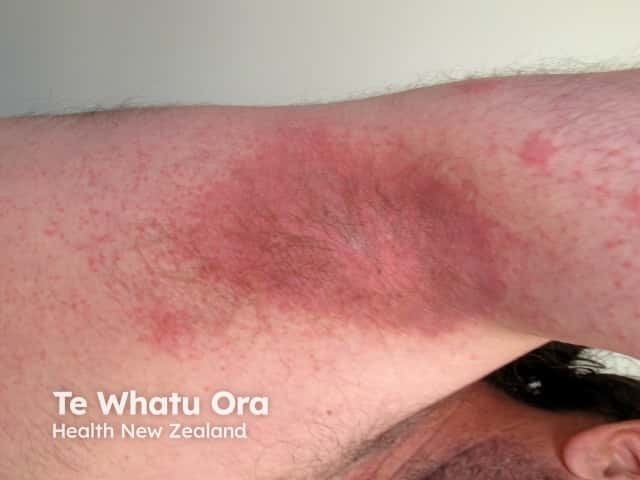
SDRIFE
What are the complications of symmetrical drug-related intertriginous and flexural exanthema?
Symmetrical drug-related intertriginous and flexural exanthema has not been reported to be associated with any complications.
How is symmetrical drug-related intertriginous and flexural exanthema diagnosed?
Symmetrical drug-related intertriginous and flexural exanthema is usually a clinical diagnosis with the following suggested diagnostic criteria:
- Follows exposure to a systemic drug, either on the first occasion or after a repeated dose
- Sharply demarcated erythema of the buttocks and/or V-shaped erythema of the inguinal area
- Involvement of at least one other flexural site
- Symmetrical distribution
- Absence of systemic symptoms.
Skin biopsy shows nonspecific inflammation consisting of a superficial perivascular mononuclear cell infiltrate.
Patch tests applied to a previously affected area several weeks after recovery may show a positive reaction to the causative agent in 50% of cases. An oral challenge with the suspected drug (drug provocation test) will trigger the same skin reaction in the majority of cases.
What is the differential diagnosis for symmetrical drug-related intertriginous and flexural exanthema?
Differential diagnoses for symmetrical drug-related intertriginous and flexural exanthema can include:
- Contact dermatitis
- Acute generalised exanthematous pustulosis (AGEP)
- Drug hypersensitivity syndrome (DRESS)
- Flexural psoriasis.
What is the treatment for symmetrical drug-related intertriginous and flexural exanthema?
Treatment of symmetrical drug-related intertriginous and flexural exanthema requires the cessation of the suspected drug. Topical steroids may hasten resolution of the erythema.
What is the outcome for symmetrical drug-related intertriginous and flexural exanthema?
Symmetrical drug-related intertriginous and flexural exanthema is a benign self-limited reaction that resolves when the offending drug is withdrawn. However, it will usually recur on re-exposure to the causative drug.
Appendix: What drugs are reported to cause symmetrical drug-related intertriginous and flexural exanthema?
Alphabetical listing of agents reported to trigger SDRIFE (note this list may not be complete)
- 5-fluorouracil
- Allopurinol
- Aminoglycoside antibiotics
- Aminophylline
- Amoxicillin
- Aspirin
- Azithromycin
- Barium sulfate
- Beta-lactam antibiotics
- Bufexamac acid
- Celecoxib
- Cephalosporin antibiotics
- Cetuximab
- Cimetidine
- Clarithromycin
- Clindamycin
- Clozapine
- Codeine
- Corticosteroids
- Cotrimoxazole
- Doxycycline
- Erythromycin
- Ethinylestradiol/etonogestrel
- Ethyl loflazepate
- Etoricoxib
- Fluconazole
- Heparin
- Hydrochlorothiazide
- Hydroxyurea
- Hydroxyzine
- Infliximab
- Iomeprol
- Iopamidol
- Ioversol
- Itraconazole
- Mefenamic acid
- Metals (gold, mercury, nickel)
- Metronidazole
- Mitomycin C
- Mosapride
- Naproxen
- Nefopam
- Nystatin
- Omeprazole
- Oxycodone
- Paracetamol
- Penicillin antibiotics
- Pioglitazone
- Pristinamycin
- Pseudoephedrine
- Ranitidine
- Remdesivir
- Risperidone
- Rivastigmine
- Roxithromycin
- Secnidazole
- Tacrolimus
- Telmisartan
- Terbinafine
- Valaciclovir
- Varenicline
Bibliography
- Aquino M, Rosner G. Systemic contact dermatitis. Clin Rev Allergy Immunol. 2019;56(1):9–18. doi:10.1007/s12016-018-8686-z. PubMed
- Ardern-Jones MR, Lee HY. Benign cutaneous adverse reactions to drugs. In: Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D (eds). Rook’s Textbook of Dermatology, 9th edn. Wiley Blackwell, 2016; 118.5–6. Journal
- Arnold AW, Hausermann P, Bach S, Bircher AJ. Recurrent flexural exanthema (SDRIFE or baboon syndrome) after administration of two different iodinated radio contrast media. Dermatology. 2007;214(1):89–93. doi:10.1159/000096920. PubMed
- Bulur I, Keseroglu HO, Saracoglu ZN, Gönül M. Symmetrical drug-related intertriginous and flexural exanthema (Baboon syndrome) associated with infliximab. J Dermatol Case Rep. 2015;9(1):12–4. doi:10.3315/jdcr.2015.1190. PubMed
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome?. Contact Dermatitis. 2004;51(5-6):297–310. doi:10.1111/j.0105-1873.2004.00445.x. PubMed
- Karadag AS, Ozlu E, Akdeniz N, et al. Oral mucosal involvement and petechial lesions: a SDRIFE case with unusual findings. Cutan Ocul Toxicol. 2016;35(2):157–9. doi:10.3109/15569527.2015.1067227. PubMed
- Nespoulous L, Matei I, Charissoux A, Bédane C, Assikar S. Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) associated with pristinamycin, secnidazole, and nefopam, with a review of the literature. Contact Dermatitis. 2018;79(6):378–80. doi:10.1111/cod.13084. PubMed
- Tan SC, Tan JW. Symmetrical drug-related intertriginous and flexural exanthema. Curr Opin Allergy Clin Immunol. 2011;11(4):313–18. doi:10.1097/ACI.0b013e3283489d5f. PubMed
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12(3):171–80. doi:10.2165/11539080-000000000-00000. PubMed
