Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
UVA photo(chemo)therapy
Created 2007.
Learning objectives
- Describe a fluorescent low-pressure mercury type of PUVA unit
- Outline UVA1 treatment
- List the indications for PUVA
- Outline the pharmacology of 8-methoxypsoralen
- Describe mechanism of action of PUVA on skin diseases
- List safety measures for PUVA
- Describe how to measure minimal phototoxic dose
- Describe dose regimen for oral PUVA
- Describe dose changes if PUVA results in erythema
- Describe dose changes if PUVA treatment is interrupted
- List supplementary treatment that can be used for psoriasis and atopic dermatitis
- List complications of PUVA
- Describe topical PUVA
UVA and PUVA
Ultraviolet-A (UVA) is the name given to the waveband of electromagnetic radiation ranging from 320-400nm. In New Zealand there is 100 times as much UVA as UVB but it has less energy. Solar UVA contributes to sunburn (10%), sun damage and skin cancer.
UVA bulbs are used in sun beds because UVA promotes tanning but is less likely to result in erythema than UVB. However, on its own UVA is ineffective in treating psoriasis and less useful than UVB for atopic dermatitis.
UVA combined with a psoralen photosensitising agent (8-methoxypsoralen or 5-methoxypsoralen) is known as PUVA or photochemotherapy. The combination is more effective than UVB in clearing or controlling a variety of skin diseases. PUVA is available at the larger public hospitals and some private dermatologists’ offices in New Zealand for treating severe skin diseases.
A modified form of PUVA is the combination of oral or topical tripsoralen and exposure to sunlight, but this can be hazardous and is not generally recommended.
Another form of photochemotherapy combines UVB with psoralen and is known as PUVB. Narrowband-UVB combined with 8-methoxypsoralens has recently been shown to be as effective as PUVA but to date there is little experience with PUVB and it will not be considered further in this course.
PUVA units in New Zealand are full body cabinets containing 6' 100W fluorescent low-pressure mercury bulbs. UVA lamps (TL100/209R) have black markings (e.g. FS72T12BLHO). Most units have 36 or 48 bulbs, and may be the same cabinet as BB-UVB and rarely NB-UVB bulbs. Hand and foot units contain 2' bulbs.
Markings on UVA bulbs 
Modern units have integrated dosimetry and the time to deliver the correct dose is automatically calculated. For older units, irradiance is checked manually prior to treatment using a specific UVA dosimeter and the treatment time for a specific dose determined according to a spreadsheet.
Small PUVA unit for hand/foot treatment 
UVA1
UVA-1 is the name given to the waveband of electromagnetic radiation ranging from 340-400nm while filtering out lower wavelengths.
- Low dose UVA1 refers to 10-20 J/cm2 per single dose
- Medium dose UVA1 refers to 50-60 J/cm2 per single dose
- High dose UVA1 refers to 130 J/cm2 per single dose
The long-term risks of high dose UVA1 are uncertain so no more than 10-15 treatments per cycle and two cycles per year are recommended. It appears to be effective in severe acute atopic dermatitis, urticaria pigmentosa, scleroderma and granuloma annulare.
There are to date no UVA1 units in New Zealand.
Daavlin UVA1 unit
Hand puva 
Indications for PUVA
PUVA is used for more severe long-lasting and resistant disease. Psoriasis or eczema with thick plaques or affecting palms and soles may resist UVB but respond well to PUVA. Patients may change to PUVA after a trial of UVB or commence on PUVA immediately.
As the erythema action spectrum for PUVA has a steep curve, slight increments in dose may result in severe burning. PUVA may be inadvisable for very fair skinned patients because of this risk.
Because of the greater likelihood of photoageing and carcinogenesis, PUVA should not be used in children. It is complicated treatment and should only be used in patients who are able to comprehend and comply with instructions.
Psoralen requires hepatic metabolism and renal excretion therefore should be used in great care in those with hepatic or renal disease. It is not teratogenic, but like other medications should be avoided in pregnancy if possible. It is excreted in breast milk so breast-feeding mothers should not receive PUVA.
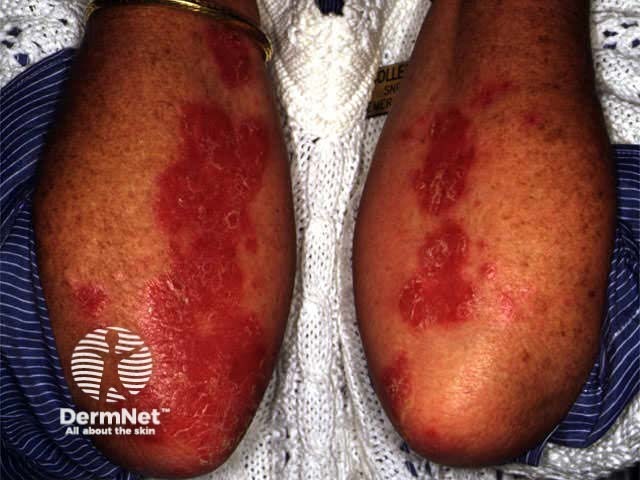
Effect of PUVA on psoriasis
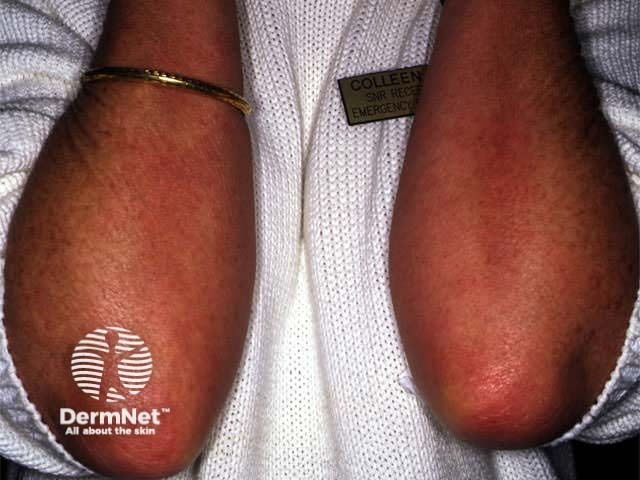
Effect of PUVA on psoriasis
Psoralen
Psoralens are a group of natural furocoumarins, commercially derived from Ammi Majus, a plant found in Egypt. They are present in celery, carrots, parsley, parsnip and other vegetables.
8-MOP capsules

8-MOP lotion

8-MOP paint
| Chemical Name | Trade Name | Use |
|---|---|---|
| 8-MOP Methoxsalen |
Oxsoralen 10mg | Used for oral PUVA |
| Puvapsoralen lotion / paint | Obtained from UK Used for bathwater soaks |
|
| 5-MOP Bergapten |
Psoraderm-5 20mg | Available under Section 29, full cost borne by patient for oral treatment. Indications:
|
| TMP Trimethylpsoralen Trioxsalen |
Tripsor 0.5 mg/5ml | Registered medicine on Drug Tariff Used for oral treatment Small supplies of topical Tripsor Lotion available for localized treatment |
The precise dose of psoralen delivered to the skin after oral ingestion cannot be determined, and serum levels are not routinely measured. However, higher concentrations cause a greater degree of phototoxicity; lower concentrations may prove ineffective.
Peak serum methoxsalen levels vary > 50-fold between patients, and are greater if taken when fasting. The peak serum and skin level is from one to 6 hours after ingestion. Carbamazepine, phenytoin and phenobarbitone reduce the level of methoxsalen. Systemic methoxsalen induces liver enzymes so that it may significantly increase serum levels of concomitant caffeine or theophylline.
For oral 8-MOP, between 0.4 and 0.6 mg/kg body weight is usually effective, although some may need more, and others less. For 5-MOP, double this dose is necessary (1 -1.2 mg/kg body weight). Poor response to PUVA can be due to inadequate absorption of the drug or a delay prior to peak levels of psoralen in the skin.
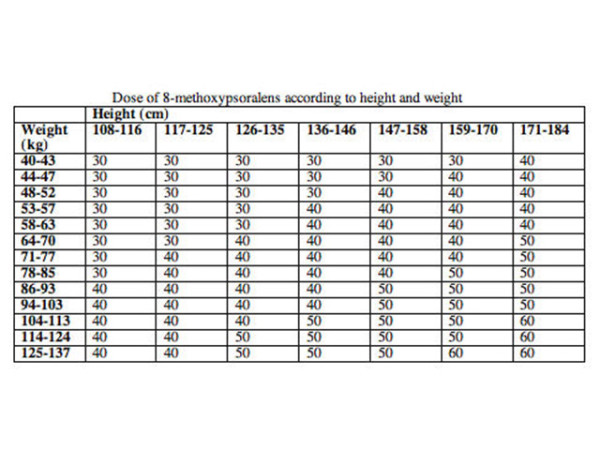
Dose per square metre of body surface area is more accurate, as in the table below.
Mechanism of action
Psoralen interacts with ultraviolet radiation in the epidermis to form DNA photo-adducts. Maximum activity occurs at a wavelength of 325 nm. The resulting photoproduct:
- Slows down keratinocyte proliferation
- Suppresses the cutaneous immune reaction
- Affects melanocytes, fibroblasts and endothelial cells
The main effects of PUVA on normal skin are inflammation (phototoxicity) and melanogenesis (tan). Inflammation reaches a peak 48-96 hours after exposure and lasts days to weeks. It affects both the epidermis (like UVB) and the dermis. It manifests clinically as erythema, oedema and in severe cases, blistering. To avoid inflammation from therapeutic PUVA, accurate dosimetry is necessary.
Melanogenesis due to PUVA is similar to that caused by UVB, i.e. melanin is deposited throughout the epidermis. The extent of melanogenesis is very variable. Deep tan blocks out UVA and can prevent adequate therapeutic effect.
UVA without psoralen (as in tanning booths) results in melanogenesis in the basal layer of the epidermis only. This protects less well against further burning.
Patient safety
- See UVB Phototherapy
- Methoxsalen is generally taken fasting, but if not, patients should be consistent with diet
- The methoxsalen dose should be the same each treatment, but if there is inadequate response and no nausea or tan, the methoxsalen can be increased by 10mg
- UVA exposure should be at a consistent interval after ingestion of methoxsalen, at the same time of day
- Patients must avoid sun exposure even behind window glass for 12 hours after ingesting methoxsalen.
- They should wear covering clothing and apply broad-spectrum sunscreens if outdoors.
- They must wear wrap-around UVA-blocking sunglasses until nightfall of the treatment day, outside whatever the weather and inside under unshielded fluorescent lamps.
- Never treat within 48 hours of previous treatment.
- Protect breasts and buttocks or other untanned areas by shielding for part of treatment time.
- They should not miss treatments. Development of tan and thickened skin will markedly reduce the efficacy of PUVA.
- All patients should have regular reviews by their dermatologist.
Minimal phototoxic dose
The minimal phototoxic dose (MPD) is typically measured as follows.
- 0.5 mg/kg of methoxsalen is ingested 2 hours prior to exposure.
- A template with four or five 1 to 2-cm squares is applied to a non-sun exposed area (often volar forearm or buttocks). The Diffey device is convenient: the templates block the radiation by nil, a half, three-quarters and seven-eighths.

Diffey device for MPD testing

Diffey device for MPD testing

Diffey device for MPD testing
- Surrounding skin is protected from exposure.
- The squares are exposed to a geometric series of increasing doses of UVA according to estimated skin type, using a 2' source (e.g. 1, 2, 4, 8 J/cm2).
- Test sites are outlined with a skin marker so they can be identified.
- The response is measured 72-96 hours later. The MPD is the lowest dose of UVA radiation that produces pink erythema with distinct borders.
- The test may be repeated using a different range of test doses if either all or none of the test sites have erythema.
- Measure MPD again if response to treatment is poor, there is a deep tan, unexpected phototoxicity or change in dose of psoralen.
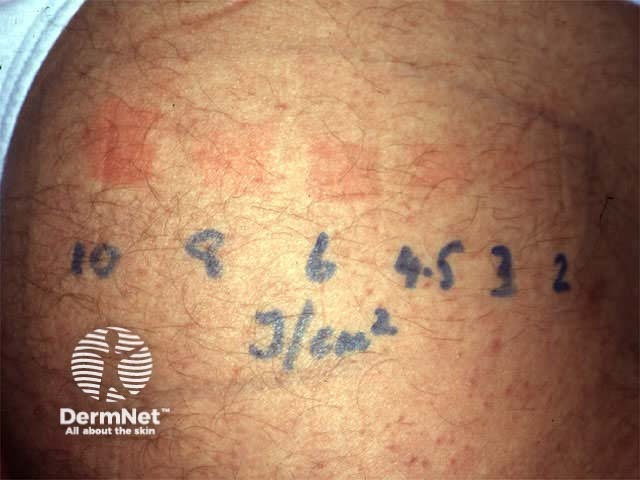
Results of MPD testing
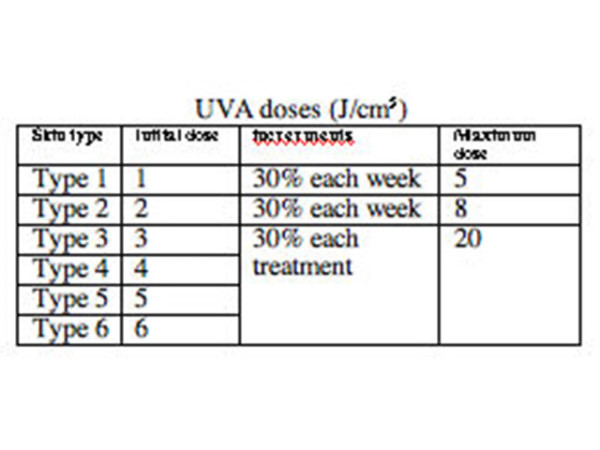
Dose regimen for UVA
To establish the appropriate starting dose of UVA, you can:
- Determine the MPD; the initial dose is 70% MPD, or,
- Select dose according to estimated skin type
- If obese, assume one skin type less as irradiance will be greater as the patient's body is closer to the bulbs
- Treat skin type 1 and 2 twice weekly to reduce chance of erythema, and types 4-6 three times weekly
- Slowly increase the doses with increments each week
Erythema
The development of any phototoxic erythema precludes further dose increments. It may be very difficult or impossible to treat skin phototype 1 patients with PUVA.
The patient should be examined prior to each treatment. Erythema may be localised or generalised. Localised erythema can be protected allowing incremental exposures to the rest of the body, as scheduled, but generalised erythema should result in changes to the schedule.
- No erythema: dose according to schedule
- Faint erythema (pink) or mild itch: hold at previous dose
- Apply emollients to dry and itchy skin
- Definite or tender erythema (pink) or significant itch: withhold treatment until resolved (may take weeks to clear and can be very distressing to the patient).
- Treat with topical steroids, aspirin, cool compresses
- Oral antihistamines may help sleeping
- Oral amitriptyline may reduce itch
- On recommencing, start with lower dose and increments should be minimal
Inform dermatologist of any significant phototoxic erythema and arrange for examination.
Tanning may necessitate a more aggressive UVA regime.
Treatment interruptions
If treatment is interrupted, UVA doses may need to be reduced to the starting dose or as follows:
One week: hold at previous dose
2 weeks: reduce dose by 25%
3 weeks: reduce dose by 50%
4 weeks or more: restart schedule
End of course
A course is completed when the skin disease has cleared or according to a pre-determined maximum number of treatments, commonly 30. Treatment may also be discontinued if there have been unresolved side effects or lack of compliance.
Maintenance therapy is not generally recommended but may be necessary for severe disease or for those who relapse rapidly after stopping treatment. Using a constant dose of methoxsalen and UVA, reduce frequency to weekly for a month then alternate weeks for a month or so. In very rare cases monthly treatment may continue but as tanning is likely to lessen, erythema may arise. Doses should be reduced by 25% per treatment. Occasional a further clearance course becomes necessary if disease flares despite maintenance.
Plaques of psoriasis should be thinning by 6 treatments, by 20 treatments the psoriasis should be 75% improved and it should be 95% clear by 30 treatments. Consider testing MPD and/or increasing the methoxsalen dose in patients who fail to clear or substantially improve with PUVA; if so, hold the UVA dose for one or two exposures. Other treatment options should be considered.
PUVA may be recommenced on relapse, depending on the severity of the disease, other treatment options and individual factors. If there is a significant tan, PUVA should be withheld until it has disappeared (at least 2 months).
Supplementary treatment
Some areas of skin disease may prove more resistant to PUVA than others, often because of prior sun exposure. Commonly, in patients with psoriasis, additional UVA is delivered to the lower limbs, nails and hands starting after about the 6th treatment. After the whole-body dose has been given, the patient dresses up to protect the rest of the body from further exposure. An extra 25-50% of the day’s dose is delivered to the resistant sites.
Many patients are treated with additional topical or systemic therapy. In some cases additive benefit has been demonstrated by clinical trials. For example, adding emollients, coal tar ointment or calcipotriol ointment and/or oral acitretin or methotrexate can reduce the number of PUVA treatments required to clear psoriasis. Occasionally UVB is added.
Emollients applied to scaly skin immediately before exposure to UVA must not act as sunscreens. Coconut oil is satisfactory. Peanut oil should be avoided because of the risk of anaphylaxis.
Complications of treatment
Acute complications
- Anorexia / nausea / headache / dizziness
- Take methoxsalen with food
- Split dose into 3 and take at 15-minute intervals
- Treat later in the day
- Reduce dose of methoxsalen by 10mg
- Pre-treat with antiemetic
- Change to 5-MOP (bergapten)
- Rarely: asthma, hepatitis, drug fever, rash, photo-onycholysis, ankle oedema, hypertrichosis
- Acute phototoxicity: tender erythema &/or deep pruritus for several weeks or longer
- Avoid this!
- Anti-inflammatories such as aspirin or indomethacin reduce pain and severity especially if started immediately after exposure
- Topical steroids applied early reduce pain and severity
- Emollients applied frequently reduce itching
- Ocular toxicity: photokeratitis causing grittiness, pain, photophobia, tearing and blepharospasm
- Frequent application of artificial tears sooth discomfort
- Polymorphous light eruption
- Irritable erythematous papules on skin that is not usually sun-exposed
- Treat with emollients and topical steroids. Rarely, systemic steroids are necessary for a severe reaction.
- Continue PUVA; oral prednisone may be necessary.
- Herpes simplex (cover and treat with aciclovir)
- Worsening skin disease
- Koebnerisation from phototoxicity is treated with more PUVA
- If due to photo-aggravation, PUVA may have to be discontinued
- Consider other reasons for flare-ups
- Consider other treatment options
Long-term complications
- Photo-ageing:
- Wrinkling, PUVA freckling, xerosis, telangiectasia, elastosis, white macules, skin atrophy, cataracts
- Skin cancer:
- Solar keratoses, squamous cell carcinoma (SCC) (x10) &rt;&rt; basal cell carcinoma (BCC) and melanoma.
- Risk of SCC is greater if >200 treatments, especially for skin type 1 and 2 and if there are other predisposing factors such as excessive sun exposure
- Genital skin cancer risk is alarming (x286): always shield genitalia.
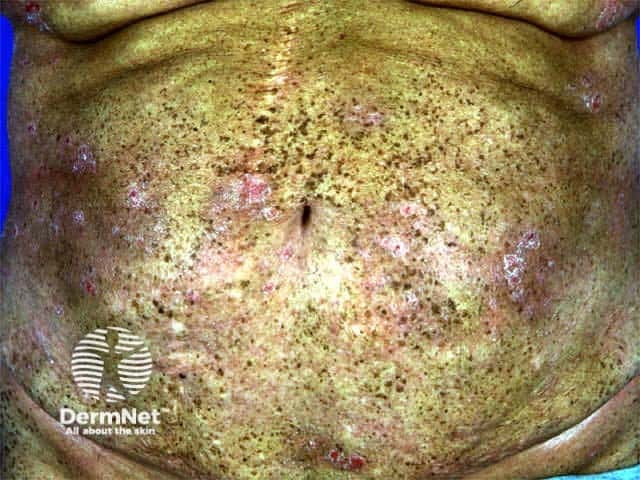
PUVA freckles
Topical psoralen
Psoralen can be applied to small areas as a cream or lotion or the area to be treated can be soaked in a dilute solution 3mg/l (bathwater PUVA). The bathwater solution distributes the psoralen evenly within the skin. This is particularly useful for treating hand and foot dermatoses but some centres use bathwater PUVA for generalised skin disease to reduce nausea. Topical psoralens are extremely photosensitising, therefor very low doses of UVA must be used.
If topical trioxsalen is used, exposure to UVA should be 10 minutes after application using very low doses (starting at 0.1 J/cm2).
Bathwater soaks in methoxsalen solution should be for 15 minutes, followed by exposure to UVA 15 minutes later using similar dose regimen to oral PUVA.
Unsightly pigmentation may arise especially in darker skin types. Phototoxic reactions are quite common and can include blistering especially on areas of friction. Photosensitivity may persist for several days so patients must protect the treated areas from exposure to natural sunlight.
Topical PUVA
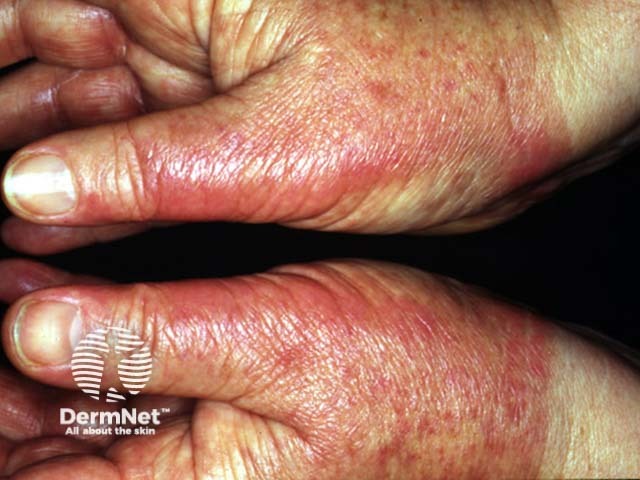
Phototoxic erythema from topical PUVA
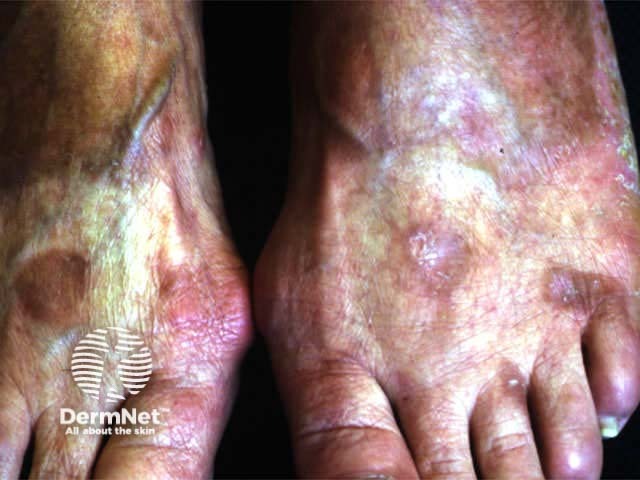
Marked pigmentation due to topical PUVA
Activity
Create a proforma to record PUVA patient treatment details and monitor safety.
References
On DermNet
Information for patients
