Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Topical formulations — extra information
Topical formulations
Author: Dr Amanda Oakley, Dermatologist, Hamilton, New Zealand, 2010. Updated in February 2016.
Introduction
Quantity
Vehicles used
Special circumstances
How to use
General principles for topical formulations
Topical formulations are applied directly to the skin. Advantages of this include:
- An increased dose of medication is applied where it is needed
- There are reduced side effects and toxicity to other organs compared to systemic medications.
Disadvantages of topical formulations include:
- They can be time-consuming to apply
- At times, the regimen can be complicated, especially if several different formulations have been prescribed
- The applications may also be messy or uncomfortable.
Topical formulations are made up in a vehicle, or base, which may be optimised for a particular site of the body or type of skin condition. The product may be designed to be moisturising or to maximise the penetration of an active ingredient, often a medicine, into or through the skin.
The amount of the active ingredient which is absorbed through the skin depends on the following factors:
- Thin skin absorbs more than thick skin — skin thickness varies with body site, age and the specific skin disorder
- Skin barrier function — this may be disrupted by dermatitis, ichthyosis, and keratolytic agents (such as salicylic acid), so it may absorb more medication than intact normal skin
- The absorption of the active ingredient is greater where there is occlusion, such as in the skin folds, under dressings, or when a greasy, ointment formulation is used
- Small molecules are more easily absorbed through the skin than large molecules
- Lipophilic compounds are better absorbed than hydrophilic compounds
- Higher concentrations of the active ingredient may penetrate more than lower concentrations
- Other ingredients in the formulation may interact to increase or reduce potency or absorption rates.
Minor differences in the formulation may make surprising differences to the effectiveness of a topical medication.
Quantity of topical formulations
How much topical medication to prescribe can challenge the most experienced dermatologist. It depends on:
- The vehicle
- The thickness of the application
- The total area to be treated
- The frequency of the application
- The duration of the treatment course.
Expect 1 g of cream to spread out over a 10-cm2 area of skin; an ointment spreads a little further. The fingertip unit (0.5 g) is a guide to the amount of a cream or ointment needed to treat an area for a certain time. One fingertip unit covers one side of 2 flat hands, and one gram covers both sides of the patient’s two hands.
It takes 20–30 g of cream or ointment to cover an adult's total body once.
Vehicles used for topical formulations
Topical formulations contain an active ingredient, often a medication or drug or botanical, and a vehicle. The vehicle usually contains water, oil, alcohol or propylene glycol mixed with preservatives, emulsifiers, absorption promoters and fragrances.
The table below describes different formulations. Manufacturers interpret the definitions in various ways so a similar preparation might be called lotion, gel or cream.
Solution |
Water or alcoholic lotion containing a dissolved powder. |

|
||
Lotion |
Usually considered thicker than a solution and more likely to contain oil as well as water or alcohol. A shake lotion separates into parts with time so needs to be shaken into suspension before use. |

|
||
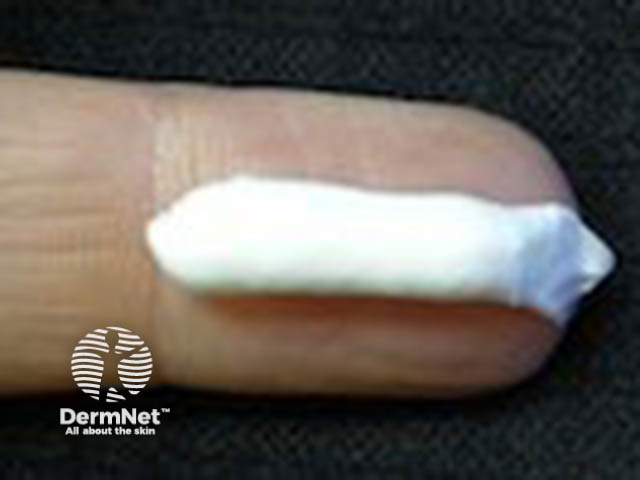
Cream |
Thicker than a lotion, maintaining its shape, for example, a 50/50 emulsion of oil and water. Requires preservative to extend shelf life. Often moisturising. |
|
||
Ointment |
Semi-solid, water-free or nearly water-free (80% oil). Greasy, sticky, emollient, protective, occlusive. No need for preservative, so contact allergy is rare. May include a hydrocarbon (paraffin), wool fat, beeswax, macrogols, emulsifying wax, cetrimide or vegetable oil (olive oil, arachis oil, coconut oil). |

|
||
Gel |
Aqueous or alcoholic monophasic semisolid emulsion, often based on cellulose and liquefies upon contact with skin. Often includes preservatives and fragrances. |

|
||
Paste |
A concentrated suspension of oil, water and powder. |

|
||
Aerosol foam or spray |
A solution with pressurised propellant. |

|
||
Powder |
Solid, for example, talc (a mineral) or corn starch (vegetable). |

|
||
Solid |
Antiperspirant or sunscreen stick. May melt on reaching body temperature (eg, a suppository). |

|
||
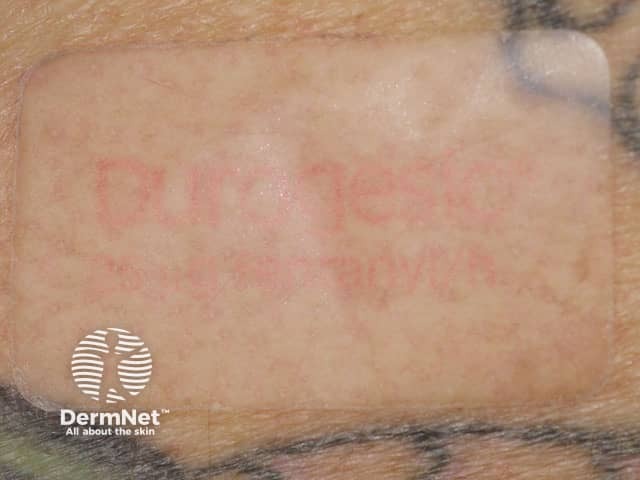
Transdermal patch |
Drug delivery system allows precise dosing: includes an adhesive. |
|
||
Other terms used by cosmetic and pharmaceutical manufacturers include emulsion, paint, suspension, milk, syrup, collodion, balm and mist. Formulae may have mixed ingredients with more than one type of vehicle.
Factors in the choice of the vehicle or base for a topical medication include the nature of the skin complaint and its site.
When a pharmacist makes up a mixture, it is extemporaneously compounded. The crude ingredients (often natural in origin) are called galenicals. They may be added to a vehicle or a brand-name product.
How does the nature of the dermatosis influence choice of topical formulation?
- For wet or oozy skin conditions — creams, lotions, and drying pastes are most suitable.
- For dry, scaly skin conditions — ointments and oils are appropriate.
- For inflamed skin — use wet compresses and soaks followed by creams or ointments.
- Cracks and sores — treat with bland applications; avoid alcohol and acidic preparations.
How does the site of the skin problem influence choice of topical formulation?
- Palms and soles — an ointment or cream may be preferred.
- Skin folds — use a cream or a lotion (ointments are too occlusive for these sites)
- Hairy areas — a lotion, solution, gel, or foam is usually best.
- Mucosal surfaces — take care to prescribe non-irritating formulations to avoid irritating eroded surfaces.
Special circumstances
Topical formulations for newborn babies
The skin barrier function of full-term newborn babies is nearly the same as in older children and adults. However, the barrier function in premature babies is markedly impaired.
The surface area of a baby is proportionally much greater than that of an adult. Organs such as the liver, kidneys, blood and central nervous system are not fully developed. This means topically applied medications can be more likely to result in side effects and toxicity.
Topical formulations during pregnancy and lactation
Like oral medicines, some topical medications may be unsafe during pregnancy and lactation. These include:
Medications are classified according to their risk. The FDA classification system is often used. Pregnancy categories used in New Zealand follow.
Category A
Drugs which have been taken by a large number of pregnant women and women of childbearing age without any proven increase in the frequency of malformations or other direct or indirect harmful effects on the fetus having been observed.
Category B1
Drugs that have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals[1] have not shown evidence of an increased occurrence of fetal damage.Category B2Drugs that have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals[1] are inadequate or may be lacking, but available data show no evidence of an increased occurrence of fetal damage.
Category B3
Drugs that have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals[1] have shown evidence of an increased occurrence of fetal damage, the significance of which is considered uncertain in humans.
Category C
Drugs that, owing to their pharmacological effects, have caused or may be suspected of causing harmful effects on the human fetus or neonate without causing malformations. These effects may be reversible.
Category D
Drugs that have caused or are suspected to have caused or may be expected to cause an increased incidence of human fetal malformations or irreversible damage. These drugs may also have adverse pharmacological effects.
Category X
Drugs that have such a high risk of causing permanent damage to the fetus that they should not be used in pregnancy or when there is a possibility of pregnancy.
Tips for using topical agents
- Topical steroids and emollients are more effective if the skin is slightly wet. So the most effective time to apply them is within 3 minutes after a bath or shower. Apply the steroid to active areas only. If you are also prescribed emollient, wait a few minutes for the topical steroid to penetrate, then apply emollient widely.
- Complaints that products sting on facial skin are common, especially if the skin is damp at the time of application. Wait 20 minutes, and the stinging is often much less troublesome.
- Stinging is common with lotions and creams and sometimes also occurs with ointments. A change of formulation rather than medicament may solve the problem. Sometimes it is best to put up with stinging, which often only lasts a few minutes because after a few applications of an effective treatment the skin heals and stinging lessens.
- Obtain a body-lotion applicator to apply the product to an area that's hard to reach, particularly the back.
- If it is difficult to squeeze out cream or ointment, cut off the end of the tube. Note that this may invalidate the expiry date and increase the chance of contamination of the product.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
References
- Bolognia, Jorizzo, et al. Dermatology e-dition. 1st Edition. Annemarie Uliasz, Mark Lebwohl. Chapter 129, Other topical medications.
On DermNet
- Topical medications for skin diseases
- Injected skin treatments
- Skin surgery
- Procedures to treat the skin
Other websites
- How to use topical steroids – Guy's and St Thomas' NHS Foundation Trust – YouTube
- How to use emollients – Guy's and St Thomas' NHS Foundation Trust – YouTube Books about skin diseases
