Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Emollients and moisturisers — extra information
Emollients and moisturisers
Author(s): Dr Libby Whittaker, Staff Writer, New Zealand (2022); Dr Amanda Oakley, Dermatologist (2016)
Previous contributors: Dr Mark Duffill, Dermatologist (1998); Isabella Boileau and Justin Mori (2019)
Edited by the DermNet content department
Introduction
Uses
Ingredients
How to use
Benefits
Side-effects and risks
What are emollients and moisturisers?
Moisturisers are products used to add moisture to the skin. Emollients are products used to soften and smooth skin (eg, lanolin, glycerol stearate). Although the terms emollient and moisturiser are often used synonymously, emollients can also be described as a specific ingredient of moisturisers.
There are often a number of available moisturisers. Options include:
- Oils
- Lotions
- Creams
- Gels
- Ointments.
Cetomacrogol and glycerine cream (sorbolene with glycerine) is a general-purpose moisturiser that is non-greasy, cheap, and available in bulk without prescription.
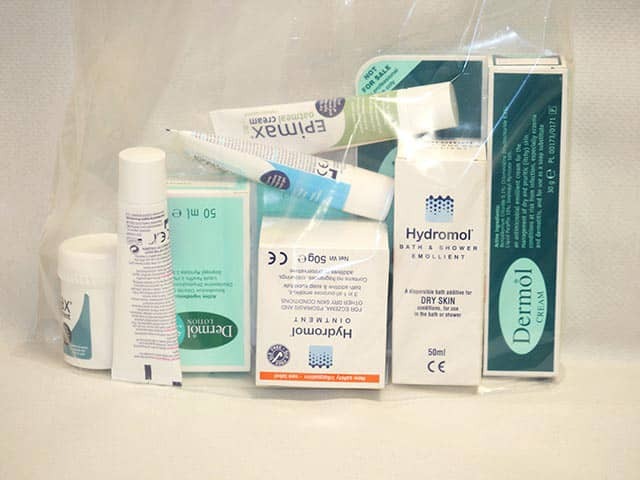
An emollient self selection bag which allows a home trial of a range of emollients, then ordering large amounts of the favoured product

A selection of emollients from the light to the very greasy - 'the right one is the one the patient likes'
What are emollients and moisturisers used for?
Uses of moisturisers include:
- Reducing skin dryness (xerosis) and scaling (ichthyosis)
- Reducing transepithelial water loss (TEWL)
- Maintenance of skin integrity, flexibility, and barrier function
- Relieving skin symptoms such as itch
- Treatment of skin conditions such as atopic dermatitis (eczema) and prevention of flare-ups
- Disguising fine lines and wrinkles
- Forming a base for other cosmetic products such as make-up.
What do moisturisers contain?
Components of moisturisers include emollients, occlusives, and humectants. Moisturisers may also contain other ingredients such as surfactants (cleansers), fragrances, and preservatives. Specially formulated products may also report antimicrobial, anti-itch, and anti-inflammatory actions.
Emollients
The are various types of emollients available, these contain oily substances such as lanolin, glyceryl stearate, and soy sterols, that soften and smooth skin by filling cracks in the skin’s outermost layer.
Occlusives
Occlusive agents provide a hydrophobic (lipophilic) layer of oil on the surface of the skin, forming a protective barrier to reduce evaporative water loss from the stratum corneum (the outermost layer of the epidermis).
Examples of occlusive ingredients:
- Petroleum jelly
- Paraffin
- Mineral oil
- Dimethicone (a component of many barrier creams used to manage hand dermatitis).
The following products are ordered from most to least occlusive:
- Ointments — best for dry, thick, scaly areas; long-lasting protective effect due to higher oil content, but some patients find them too greasy, especially on visible areas
- Creams — suitable for broad application; lighter than ointments therefore more quickly absorbed but also require more frequent application
- Lotions — often used for hairy areas such as the scalp, and/or for mild dryness elsewhere
- Oils — eg, bath oils deposit a thin layer of oil on the skin upon rising from the water. A study has demonstrated decreased skin barrier function when frequent bath oils are used in infants less that 12 months of age.
Humectants
Humectant agents are hydrophilic (‘water-loving’), therefore attract and retain water in the stratum corneum, similar to the natural hydrating factors found in corneocytes. They include:
- Glycerine
- Urea
- Alpha hydroxy acids (eg, lactic acid or glycolic acid)
- Salicylic acid.
Urea and other acidic preparations often sting if applied to scratched or fissured skin. They are also keratolytic, ie, they have a descaling or peeling effect, which is important in the management of ichthyosis.
Preservatives
Creams and lotions are prone to microbial contamination and preservatives (eg, parabens, formaldehyde-releasers, and isothiazolinones) are added to improve shelf life. Preservatives in moisturisers can lead to allergic contact dermatitis in sensitised individuals.
Other ingredients
- Anti-pruritic ingredients: include lauromacrogols (which exert a mild anaesthetic effect), menthol, and oatmeal;
- Anti-inflammatory ingredients: include chamomile, aloe vera, and shea butter.
Many additional agents may be added to a moisturiser to appeal to the consumer. Be aware that marketing claims for these ingredients in reducing the signs of skin ageing can be misleading. Large molecules such as peptides and collagen cannot penetrate through the stratum corneum.
How to use moisturisers and emollients
- To prevent skin dryness, soap cleansers and shower gels should be used sparingly, or not at all.
- Moisturising soap substitutes are a good alternative for people with dry and/or sensitive skin.
- Bath emollients are oils or emulsifiers designed to disperse in the bath, and are best used in conjunction with other moisturisers including leave-on products.
- Combinations of products are often used, eg, soap substitutes +/- bath oils, as well as leave-on products.
- Leave-on moisturising ointments, creams, and lotions are most effective when applied immediately after bathing, as water that has entered the epidermis remains there, but they can also be applied at other times.
Application frequency and quantity is dependent on the severity of the dry skin:
- Very dry skin may benefit from a greasy, occlusive moisturiser applied every couple of hours, while slightly dry skin may only need a light moisturiser at night.
- The amount of moisturiser applied per week in patients with eczema averages from 150–200 g in young children to 500 g in adults.
What are the benefits of moisturisers?
- Reduced skin dryness, leading to improved skin integrity and barrier function.
- In patients with eczema, use of moisturisers and emollients may be sufficient to control mild disease and eliminate symptoms; reduce severity of flare-ups; and reduce requirements for topical steroids.
- Therapeutic effects of moisturisers are greatest in the first 6 hours after their application; regular application 2–3 times/day may be needed to maximise benefits.
What are the side-effects and risks of moisturisers?
Irritant reactions
People with sensitive skin associated with atopic dermatitis or rosacea often describe irritant reactions to moisturisers, such as burning and stinging. If irritation is transient, the product can generally continue to be used. It should be discontinued if contact dermatitis appears (see below).
Contact allergy
Discontinue use if allergic contact dermatitis develops to a particular emollient or moisturiser. Suspected allergic contact dermatitis to particular preservatives, fragrances, or vehicles can be investigated by patch testing.
Folliculitis
Occlusive emollients can cause or aggravate acne, perioral dermatitis, folliculitis, and boils.
Thermal burns
Thermal burns from paraffin-containing ointments on clothing or bedding can be dangerous and can occur if ignited by cigarettes or naked flames.
Slips and falls
Moisturising products (bath oils in particular) can be slippery and care should be taken while getting in and out of the bath or shower to avoid falls. Use of a bath mat may minimise this risk.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).
Bibliography
- Anderson PC, Dinulos JG. Are the new moisturizers more effective? Curr Opin Pediatr 2009; 21: 486–90. DOI: 10.1097/MOP.0b013e32832cfd3b. Journal
- Danby SG, Chalmers J, Brown K, Williams HC, Cork MJ. A functional mechanistic study of the effect of emollients on the structure and function of the skin barrier. Br J Dermatol 2016; 175: 1011–9. DOI: 10.1111/bjd.14684. Journal
- Hon KL, Kung JSC, Ng WGG, Leung TF. Emollient treatment of atopic dermatitis: latest evidence and clinical considerations. Drugs Context 2018; 7: 212530. DOI: 10.7573/dic.212530. PubMed Central
- Purnamawati S, Indrastuti N, Danarti R, Saefudin T. The role of moisturizers in addressing various kinds of dermatitis: A review. Clin Med Res 2017; 115(2-3): 75–87. DOI: 10.3121/cmr.2017.1363. PubMed Central
- Ridd MJ, Redmond NM, Hollinghurst S, et al. Choice of Moisturiser for Eczema Treatment (COMET): study protocol for a randomized controlled trial. Trials 2015; 16: 304. DOI: 10.1186/s13063-015-0830-y. Journal
- Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol 2012; 26: 1045–60. DOI: 10.1111/j.1468-3083.2012.04635.x. Journal
- Varothai S, Nitayavardhana S, Kulthanan K. Review article: Moisturizers for patients with atopic dermatitis. Asian Pac J Allergy Immunol 2013; 31: 91-8. Journal
- Zuuren EJ, Fedorowicz Z, Arents BWM. Emollients and moisturizers for eczema: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol 2017; 177: 1256–71. DOI: 10.1111/bjd.15602. Journal
On DermNet
- Atopic dermatitis
- Barrier creams
- Barrier function in atopic dermatitis
- Dermatitis
- Dry skin
- Ichthyosis
- Occupational skin disease
- Soaps and cleansers
- Topical steroids
Other websites
- How to use emollients — Guy's and St Thomas' NHS Foundation Trust (YouTube video)
