Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Morbilliform drug reaction — extra information
Morbilliform drug reaction
Author: Dr Delwyn Dyall-Smith FACD, Dermatologist, Australia, 2009. Updated by A/Prof Amanda Oakley, January 2016.
Introduction
Demographics
Causes
Clinical features
Complications
Diagnosis
Treatment
Prevention
Outlook
What is morbilliform drug reaction?
Morbilliform drug eruption is the most common form of drug eruption. Many drugs can trigger this allergic reaction, but antibiotics are the most common group. The eruption may resemble exanthems caused by viral and bacterial infections.
- A morbilliform skin rash in an adult is usually due to a drug.
- In a child, it is more likely to be viral in origin.
Morbilliform drug eruption is also called maculopapular drug eruption, exanthematous drug eruption and maculopapular exanthem.
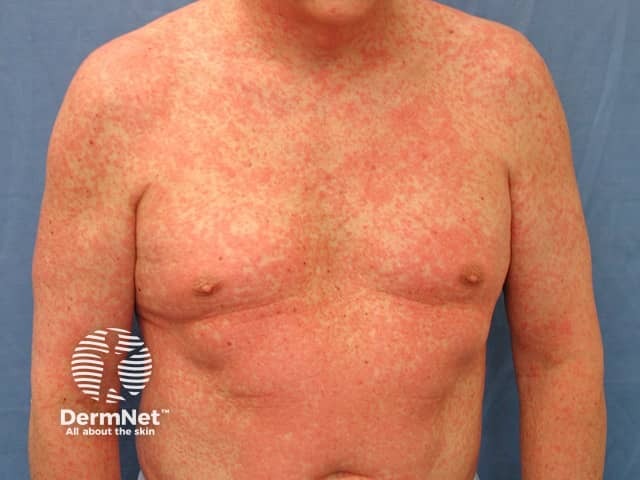
Morbilliform drug eruption
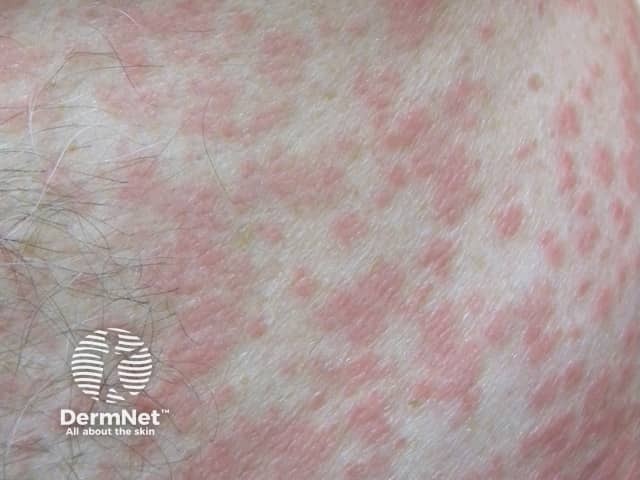
Morbilliform drug eruption
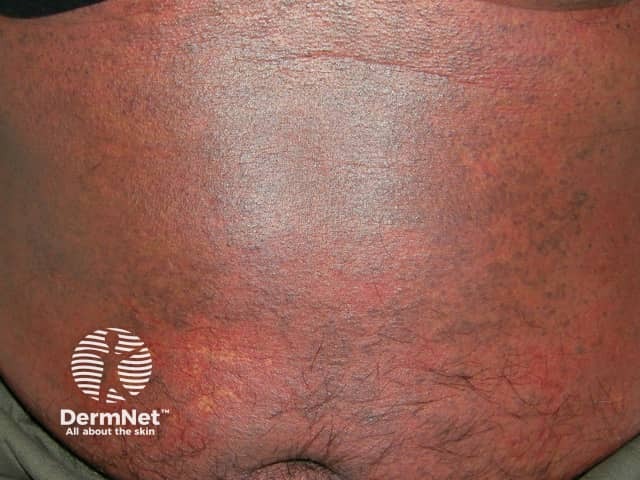
Purpuric morbilliform eruption due to thrombocytopenia
Who gets morbilliform drug eruption?
About 2% of prescriptions of new drugs cause a drug eruption. About 95% of these are morbilliform drug eruptions.
They mainly affect people prescribed beta-lactam antibiotics (penicillins, cephalosporins), sulfonamides, allopurinol, anti-epileptic drugs and nonsteroidal anti-inflammatory drugs (NSAID). Numerous other drugs have been reported to cause morbilliform drug eruptions, including herbal and natural therapies.
Predisposing factors include:
- Previous drug eruption or strong family history of drug eruptions
- Underlying viral infection, particularly acute Epstein-Barr virus (EBV, infectious mononucleosis) and human herpesvirus 6 and 7 (see also roseola, pityriasis rosea)
- Immunodeficiency including human immunodeficiency virus (HIV), cystic fibrosis, autoimmune disorders
- Multiple medications
What causes morbilliform drug eruption?
Morbilliform drug eruption is a form of allergic reaction. It is mediated by cytotoxic T-cells and classified as a Type IV immune reaction. The target of attack may be drug, a metabolite of the drug, or a protein bonded to the drug. Inflammation follows the release of cytokines and other effector immune cells.
What are the clinical features of morbilliform drug eruption?
On the first occasion, a morbilliform rash usually appears 1–2 weeks after starting the drug, but it may occur up to 1 week after stopping it. On re-exposure to the causative (or related) drug, skin lesions appear within 1–3 days. It is very rare for a drug that has been taken for months or years to cause a morbilliform drug eruption.
Morbilliform drug eruption usually first appears on the trunk and then spreads to the limbs and neck. The distribution is bilateral and symmetrical.
The primary lesion is a pink-to-red flat macule or papule.
- Annular, targetoid, urticaria-like or polymorphous morphology may occur.
- Lesions mostly blanch with pressure but may be non-blanchable (purpuric) on the lower legs.
- Discrete lesions may merge together to form large erythematous patches or plaques.
- Axilla, groin, hands and feet are usually spared.
- Paradoxical prominent rash in axillae and groins may be due to symmetrical drug related intertriginous and flexural exanthem (SDRIFE)
- Mucous membranes, hair and nails are not affected in uncomplicated drug eruptions.
The rash may be associated with a mild fever and itch. As it improves, the redness dies away and the surface skin peels off.
What are the complications of morbilliform drug eruption?
In the early phase, it may not be possible to clinically distinguish an uncomplicated morbilliform eruption from other more serious cutaneous adverse reactions (SCAR). These are:
- Drug hypersensitivity syndrome
- Stevens Johnson syndrome – toxic epidermal necrolysis (SJS/TEN)
- Acute generalised exanthematous pustulosis (AGEP)
Patients with the following symptoms/signs should be hospitalised for specialist assessment and supportive care.
- Erythroderma (whole-body involvement)
- High fever or significant malaise
- Any mucosal involvement
- Skin tenderness
- Blistering
- Pustules
- Palpable purpura
- Evidence of other organ involvement (eg liver, kidneys, lungs, blood)
How is morbilliform drug eruption diagnosed?
A strong clinical suspicion of morbilliform drug eruption depends on:
- Typical exanthematous rash
- Recently introduced medication
To identify the possible causative drug, a drug calendar, including all prescribed and over-the counter products, may be helpful. The starting date of each new drug is documented together with the onset of the rash. The calendar must extend back at least 2 weeks and up to one month. Drugs can then be classified as unlikely or likely causes based on:
- Time relative to onset of the rash
- The specific drug; some drugs can be excluded as rarely causing allergy
- Patient’s past experience with other drugs in the same class
There are no routine tests to make the diagnosis or to identify the culprit drug. Differential diagnosis includes measles, rubella, scarlet fever, non-specific toxic erythema associated with infection, Kawasaki disease, connective tissue disease and acute graft-versus-host disease.
Tests are not usually necessary if the cause has been identified and stopped, the rash is mild and the patient is well. They may include:
- Routine blood count, liver and kidney function tests, C-reactive protein
- Serology for infections that can cause similar rashes
- Possible skin biopsy, which shows interface dermatitis, mixed perivascular infiltration and other histopathological features.
Eosinophilia is supportive but not diagnostic. Further investigations will depend on clinical features, progress of the patient, and the results of the initial tests.
What is the treatment for morbilliform drug eruption?
The most important thing is to identify the causative drug and if possible, stop it. If the reaction is mild, and the drug is essential and not replaceable, obtain a specialist opinion whether it is safe to continue the drug before doing so.
- Monitor the patient carefully in case of complications.
- Apply emollients and potent topical steroid creams.
- Consider wet wraps for very red, inflamed skin.
- Antihistamines are often prescribed, but in general they not very helpful.
How can morbilliform drug eruption be prevented?
It is not possible to completely prevent morbilliform eruptions. Prescribers must be vigilant. Their incidence may be reduced by:
- Minimising prescriptions for antibiotics
- Educating the patient about the cause of their rash and the danger of re-exposure to the same medication
- Adding the reaction to the medical record alerts
What is the outlook for morbilliform drug eruption?
If the causative drug is ceased, the rash begins to improve within 48 hours and clears within 1–2 weeks.
If the drug is continued, the rash may:
- Resolve despite continued exposure to the drug
- Persist without change
- Progress to erythroderma
Reference
- Dermatology. Jean L. Bolognia, Joseph Jorizzo and Ronald Rapini. 2 volume set. 2nd Edition 2007. 2432 pages. Mosby.
- Cotliar J. Approach to the patient with a suspected drug eruption. Semin. Cutan. Med. Surg. 2007; 26:147–154. PubMed
- Mays SR, Kunishige JH, Truong E, Kontoyiannis DP, Hymes SR. Approach to the morbilliform eruption in the hematopoietic transplant patient. Semin. Cutan. Med. Surg. 2007; 26:155–162. PubMed
- Yawalkar N. Drug-induced exanthems. Toxicology 2005; 209: 131–4. PubMed
On DermNet
Other websites
- Understanding drug eruptions — DermNet e-lecture [Youtube]
- Drug eruptions — Medscape Reference
