Main menu
Common skin conditions

NEWS
Join DermNet PRO
Read more
Quick links
Botulinum toxin — extra information
Botulinum toxin
Author: Dr Ken Hiu-Kan Ip, Dermatology Registrar, Auckland City Hospital. Technical Editor: Mary Elaine Luther, Medical Student, Ross University School of Medicine, Barbados. DermNet Editor in Chief: Adjunct A/Prof. Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell. January 2020.
Introduction How it works Differences between types Age reversal Approved uses Uses Contraindications Adverse effects Other uses
What is botulinum toxin?
Botulinum toxin is an injectable muscle relaxant, commonly known by one of its trademarked brand names, BOTOX®.
Botulinum toxin is responsible for causing botulism, the paralysing and potentially fatal foodborne illness. The name botulinum is derived from the Latin word botulus (sausage) because the illness was once believed to originate from sausages. We now know that the botulinum toxin is produced by Clostridium botulinum bacteria.
C. botulinum produces eight types of toxins, labelled from A to G. Since the 1950s, research has focused on developing the muscle-relaxing properties of botulinum-A, and more recently botulinum-B, for therapeutic use.
How do botulinum toxins work?
Botulinum-A and botulinum-B block nerve signals in the muscles. In order to contract, a muscle must receive a signal from peripheral nerve endings. This signal is the neurotransmitter, acetylcholine, which binds the receptors with the muscle, initiating muscle contraction. Botulinum toxin inhibits the release of acetylcholine and therefore also inhibits muscle contraction.
More specifically, botulinum-A and botulinum-B target a group of proteins called SNARE (soluble NSF attachment protein receptor). The function of SNARE is to release neurotransmitters from their storage vesicles by helping vesicle membranes fuse with the cell membrane. Because SNARE is also involved in the release of other neurotransmitters, botulinum toxins have additional effects. For example, by preventing the release of glutamate and substance P, botulinum toxin lessens the sensation of pain.
Muscle weakness is typically seen within two to four days, and maximal weakness or paralysis is noted around ten days. The effects of botulinum toxins are not permanent, because the affected nerves sprout new endings. After around three months, a working connection between the nerve and muscle is re-established. However, an element of atrophy (muscle wasting) occurs with each injection. This incremental atrophy explains why the interval needed for a second treatment can gradually lengthen following repeated injections.
What are the differences between the different types of botulinum-A?
In New Zealand, there are three commercially available brands of botulinum-A:
- Botox® (onabotolinumtoxinA)
- Dysport® (abobotulinumtoxinA)
- Xeomin® (incobotulinumtoxinA).
Each form has a unique molecular structure but shares the same core elements of the botulinum-A. The majority of research for cosmetic uses of botulinum-A have been based on onabotolinumtoxinA.
It is important to note that there is no standardised international unit that can be used to compare or to measure the amount of botulinum toxin administered. This means the effect of one unit of Botox is different than one unit of Dysport. The approximate conversion factor is 1:2.5 to 1:5 between Botox and Dysport; and 1:1 between Botox and Xeomin.
Myobloc (rimabotulinumtoxinB) is a commercially available brand of botulinum-B. Myobloc is used mainly in other medical specialities outside of dermatology.
Can botulinum-A reverse the effects of ageing?
The appearance of ageing skin arises from a combination of factors. Rhytides (wrinkles) are caused by altered pigmentation, redundant and sagging skin, and loss of soft tissue. Rhytides caused by muscle movements are called dynamic rhytides, while those rhytides that do not change with muscle movement are called static rhytides.
As an injectable muscle relaxant, botulinum-A is effective in treating dynamic rhytides and is currently FDA-approved for the treatment of rhytides occurring in the upper face.
Forehead lines
Horizontal lines across the forehead result from contraction of the frontalis muscle. This is the facial muscle involved when you express worriedness; hence the name ‘worry lines’.
Glabella lines
The vertical lines between the eyebrows result from contraction of procerus and corrugator supercilii muscles. These are the facial muscles involved in frowning; hence the name ‘frown lines’.
Effects of botulinum toxin
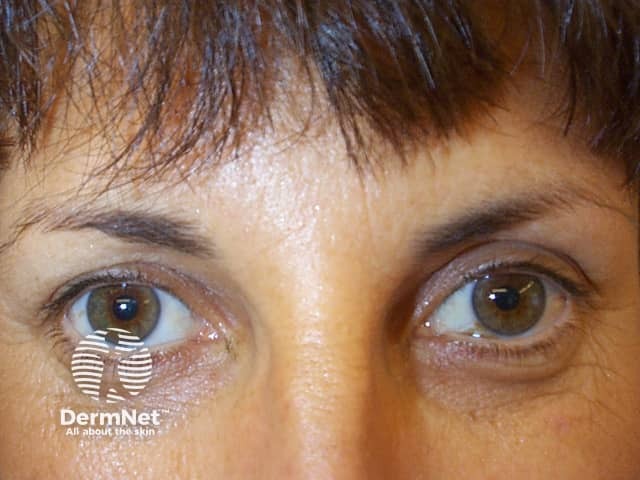
Subject relaxed prior to botulinum toxin injections
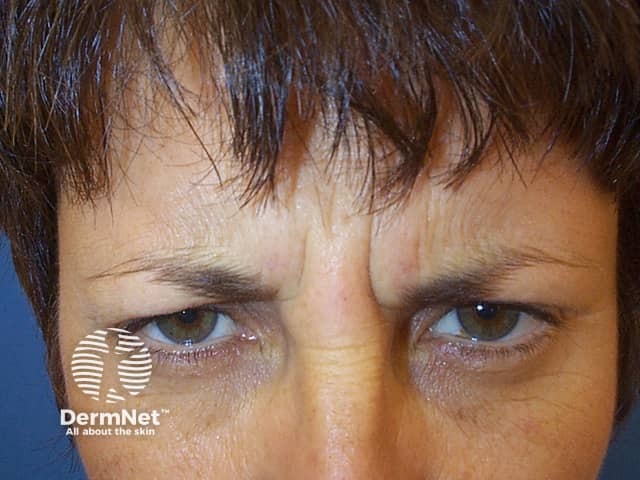
Subject frowning prior to botulinum toxin injectons
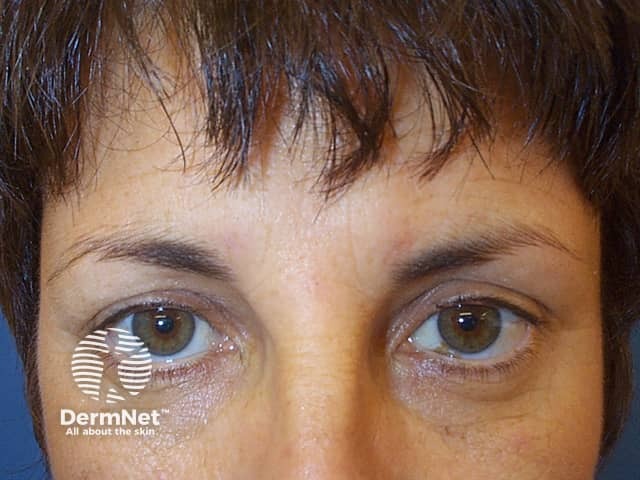
Subject relaxed after botulinum toxin injections
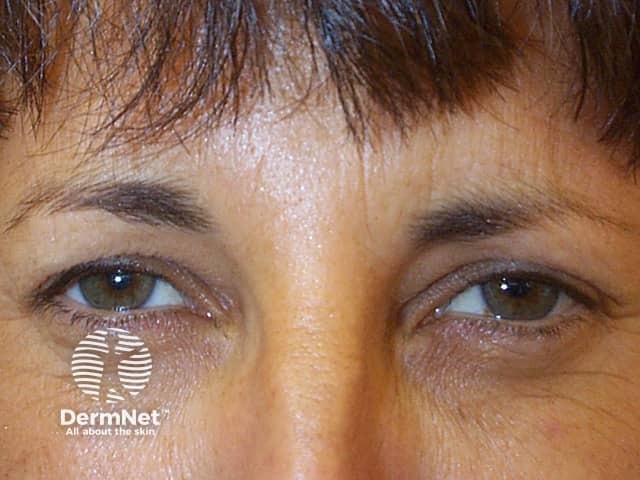
Subject attempting to frown after botulinum toxin injections
Crow’s feet
Horizontal lines branching out from the outer corners of the eyes result from contraction of the orbicularis oculi muscle. This is the facial muscle involved in the facial expression when smiling, and the resulting lines are named after their resemblance to crow’s feet.
Botulinum-A is used off-label to treat dynamic rhytides elsewhere on the face.
Botulinum toxin injections for crows' feet
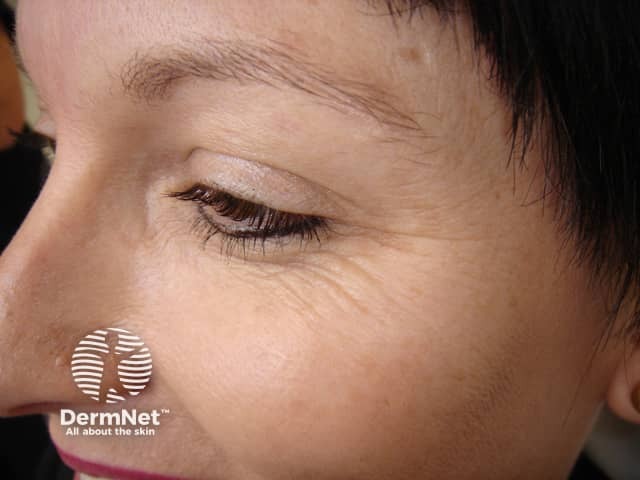
Before injection
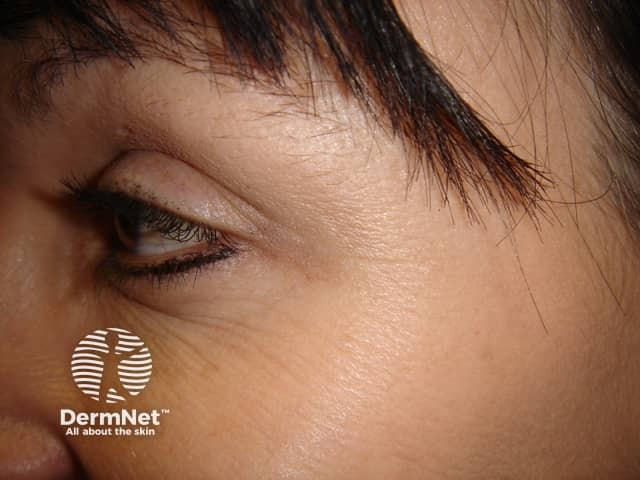
After injection
Are there other approved uses for botulinum-A?
The other major use of botulinum-A in dermatology is for treatment of localised hyperhidrosis. Hyperhidrosis is a condition of excess and uncontrollable sweating. Botulinum-A injected into the skin of the axillae and palms targets the eccrine sweat glands to prevent sweating. It is used off-label to treat the auriculotemporal syndrome.
Botulinum-A is also approved for the following uses:
- Overactive bladder causing symptoms of urinary incontinence, urgency, and frequency
- Prevention of migraines
- Blepharospasm (abnormal contraction of eyelid muscles)
- Cervical dystonia (painful spasms of neck muscles)
- Focal spasticity (localised groups of overactive muscles).
How is botulinum-A administered?
Each person’s facial musculature is unique; therefore, the approach to botulinum-A injection should be individualised. Prior to any treatment, it is important to assess for any pre-existing asymmetries or scars, for any evidence of concurrent infection, and for previous cosmetic surgeries — especially to the eyelids.
Botulinum-A is stored in vials, which must be reconstituted by mixing with sterile saline water. The mixture should be used within 24 hours in order to ensure that it does not become contaminated.
A fine needle (30 gauge) is recommended. For the treatment of dynamic rhytides, small (0.1 mL) injections are made precisely into the muscle responsible for the facial lines. Generally, injections in up to five different sites can be expected when receiving treatment for the upper face. A local anaesthetic is not usually required. The exception is for treatment of hyperhidrosis of the hands and feet, where injection into the thicker skin can cause a significant amount of discomfort.
In rare instances, the body’s immune system might respond to botulinum-A injections by producing antibodies that attempt to neutralise the effects of the toxin. The main risk factor for developing antibodies is the frequency of injections. Thus, injections generally should not be given more frequently than once every eight to twelve weeks.
What are the contraindications to receiving botulinum-A injections?
Botulinum-A is contraindicated in individuals with:
- Pre-existing medical conditions that cause muscle weakness, including motor neurone disease (amyotrophic lateral sclerosis) and myasthenia gravis
- Infection overlying the injection site
- Previous allergic or hypersensitive reactions to botulinum-A.
Botulinum-A should also be avoided during pregnancy and while breastfeeding.
Certain medications that enhance the effects botulinum-A should be avoided. These medications include tetracycline antibiotics and aminoglycoside antibiotics.
Blood thinners — including aspirin, warfarin and dabigatran — increase the risk of significant bruising from injection sites.
What are the adverse effects of botulinum-A injections?
The most common adverse effects of botulinum-A are related to the injection, rather than to the botulinum toxin itself. These adverse effects include pain, bruising, swelling, and redness at the injection site.
Bruise from botulinum toxin injections
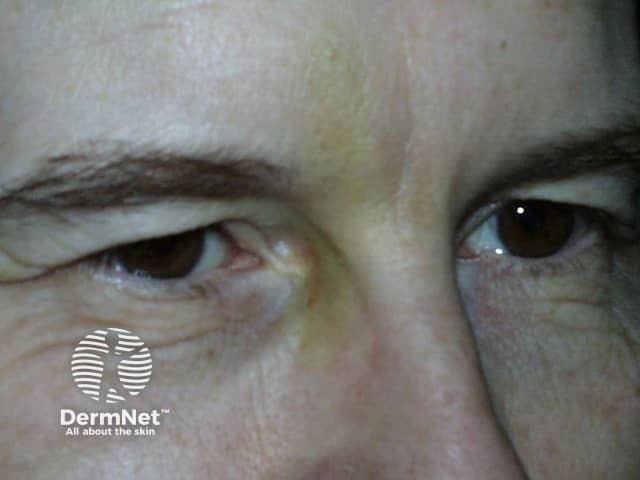
BOTOX® injections
- An uncommon side-effect of botulinum-A is the unintended weakness of the surrounding muscles (occurring in fewer than 10% of treatments). This weakness results from the spread of the toxin from the injection site into adjacent muscle groups.
- The eyelids (ptosis), brows, and lips can be affected.
- The weakness can persist for up to 12 weeks.
- Medicated α-adrenergic eye drops can be prescribed for treatment of droopy eyelids and eyebrows.
- The hand muscles can be unintentionally affected when treating palmar hyperhidrosis, resulting in temporary weakness or reduced dexterity.
- Some people note a mild headache, usually two to four hours after injection into forehead muscles, which can be treated with paracetamol (acetaminophen).
Droopy eyelid from botulinum toxin injections
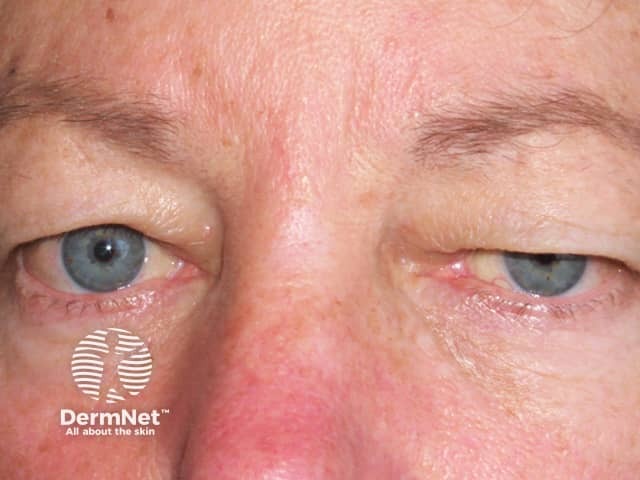
BOTOX® injections
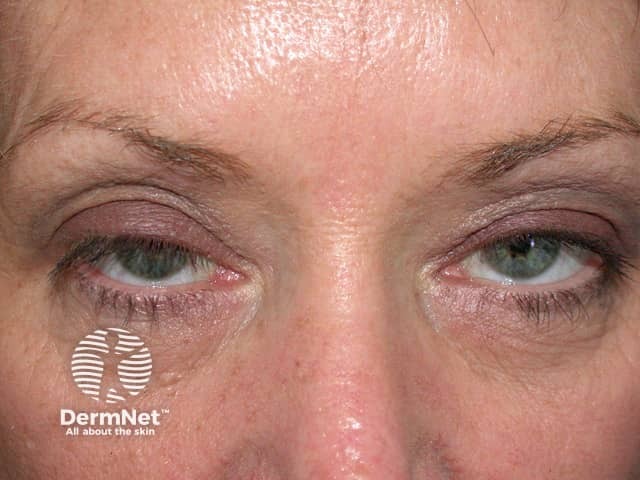
BOTOX® injections
What other uses for botulinum-A are currently being studied?
New uses for botulinum-A in dermatology are being investigated.
Keloid and hypertrophic scars
Botulinum-A injected to paralyse the muscles underlying a healing wound can reduce wound tension in keloids and hypertrophic scars, which is a major determinant of cosmetic outcome. Botulinum-A can also block nerve signals to help modulate the pain, redness, and itchiness associated with keloid scars.
Facial erythema and flushing
Mixed results have been obtained in studies for using botulinum-A to reduce facial flushing associated with menopause and rosacea.
Oily skin
Botulinum-A can reduce sebum production in the facial region, due to its effects on the arrector pili muscles.
Postherpetic neuralgia
Postherpetic neuralgia is a common complication of herpes zoster is ongoing pain after the resolution of the rash. Botulinum-A has been shown to significantly reduce this residual pain by modulating nerve signals.
Raynaud phenomenon
Botulinum-A injections into the neurovascular bundles on the hands have been reported to reduce pain in Raynaud phenomenon, which is associated with excessive blood vessel constriction when exposed to cold.
Conditions affecting skin folds
Botulinum-A has also shown promise in improving outcomes for dermatological conditions affecting skin folds, likely through modifying the moist environment to reduce maceration and infection. These conditions include hidradenitis suppurativa, benign familial pemphigus, and flexural psoriasis.
References
- Allergan New Zealand Limited. Botox Botulinum Toxin Type A Data Sheet, November 2017. Available at: medsafe.govt.nz/profs/Datasheet/b/Botoxinj.pdf (accessed 26 February 2019).
- Campanati A, Martina E, Giuliodori K, Consales V, Bobyr I, Offidani A. Botulinum toxin off-label use in dermatology: a review. Skin Appendage Disord 2017; 3: 39-56. DOI:10.1159/000452341. PubMed
- Healthcare Logistics. Dysport Data Sheet, July 2018. Available at: www.medsafe.govt.nz/profs/datasheet/d/Dysportinj.pdf (accessed 26 February 2019).
- Healthcare Logistics. Xeomin Data Sheet, January 2019. Available at: medsafe.govt.nz/profs/Datasheet/x/Xeomininj.pdf (accessed 26 February 2019).
- Wolverton SE. Comprehensive dermatologic drug therapy, Elsevier Health Sciences, 2012.
On DermNet
Other websites
- Consumer medicine information and data sheets — Medsafe New Zealand
- BOTOX® injections — Medscape Reference
- BOTOX® Injections — emedicinehealth
- Botox Facts.ca — SkinCareGuide.com
- Botulinum toxin — Therapeutics in Dermatology
- Botulinum toxin — American Academy of Dermatology
